Children with Chronic Conditions
Session: Children with Chronic Conditions 3
285 - ‘Medical-Technology’ and ‘Family-Centred-Feeding’ measures for caregivers of children with medical complexity (Co-CMC).
Friday, May 3, 2024
5:15 PM - 7:15 PM ET
Poster Number: 285
Publication Number: 285.349
Publication Number: 285.349
- NF
Nora Fayed, PhD (she/her/hers)
Associate Professor
Queen's University
Kingston, Ontario, Canada
Presenting Author(s)
Background: Children with medical complexity have high health care needs with medical-technology dependence. 'Caregiving for medical technology' and 'feeding', are important outcomes for service evaluations of caregivers of children with medical complexity (Co-CMC), but no tools were found.
Objective: To develop and test patient reported outcome measures (PROMs), of 'Medical-Technology’ and ‘Family-Centered Feeding’.
Design/Methods: A sequential mixed-method design was used. In Phase I, (item-generation), measurement content was derived from Co-CMC interviews. Co-CMC lived-experience consultants on the research team scored candidate items for criteria of i) importance, ii) comprehension, and iii) ethics, in-order to flag poor candidate items. Co-CMC consultants and authors re-worded or eliminated low scoring items, as appropriate.
Phase II, (quantitative field-testing), was conducted on a new clinical and online community-dwelling convenience sample of Co-CMC, whose children were 18-months to 21-years of age. A classical test theory analysis was conducted to test for multiple-domain research scales; while a Rasch analysis was used to test for shorter unidimensional clinical scales.
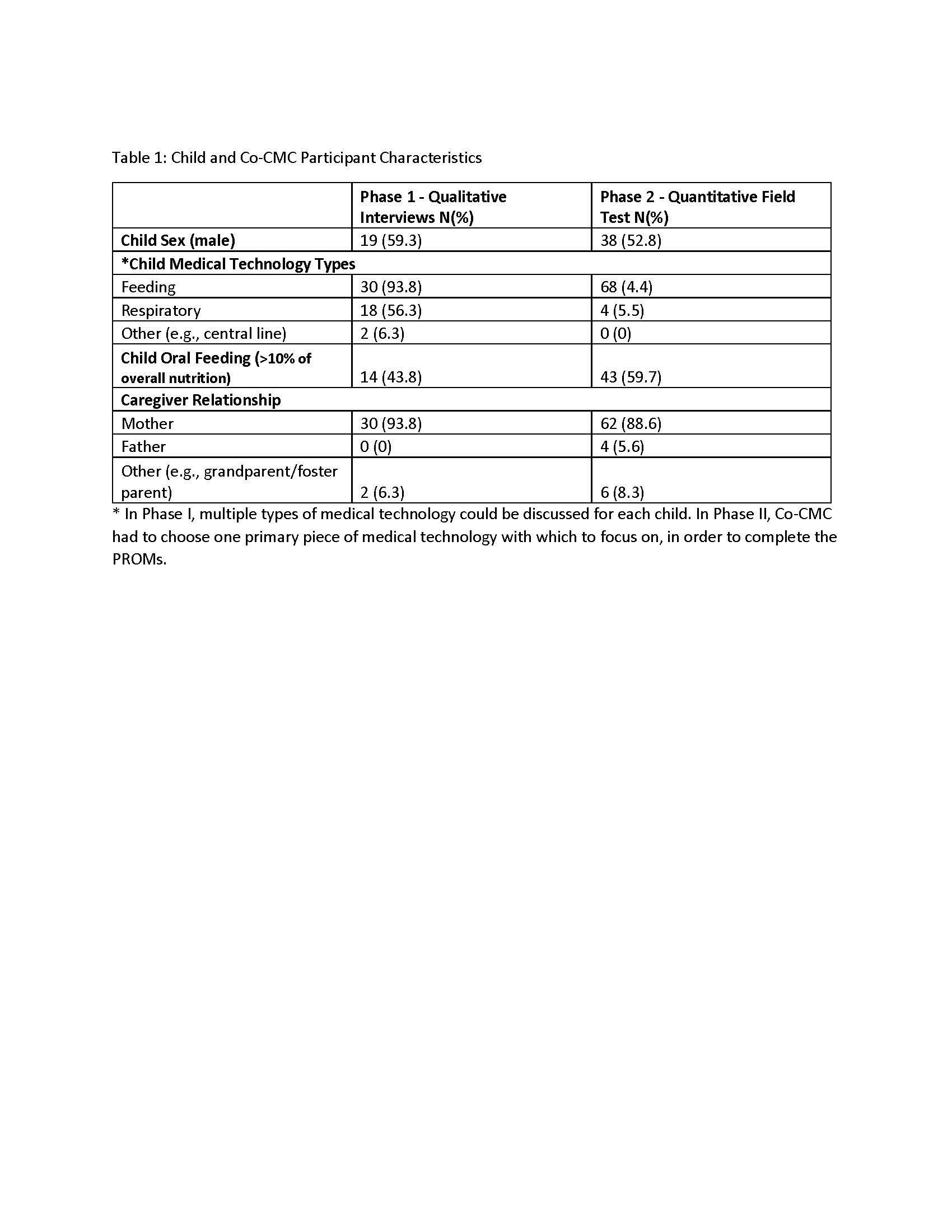
Results: Phase I, (N=32) Co-CMC of children X=8.72, (2-21) years of age, participated in the interviews, additional participant characteristics found in Table 1. Proposed multi-domain scales for the Med-Tech-PROM were: ‘Child Perspective’, ‘Family Impact, ‘Sleep’, ‘Technical Competence’, ‘Supplies/Cost’, “Daycare/School’, ‘Health-Provider’, ‘Mobility, and “Peer Acceptance’. Family-Centered-Feeding-PROM domains were: ‘Doing-Feeding’, ‘Feeling-Feeding’, ‘Family’, ‘Child’s Perspective’, ‘Mealtime’, ‘Daycare/School’ and ‘Health Provider’.
Phase II (N=72) Co-CMC completed the multi-domain scale PROM scales. Cronbach’s α=0.76-0.89, inter-item correlations 0.32-0.87 (within domains), and factor loadings of items to the proposed domain=0.62-0.93. Administration time was up to 35 minutes/PROM. Rasch-analysis results were inconclusive due to the insufficient sample size obtained by the analysis cut-off date. A shortened scale was not validated.
Conclusion(s): Multi-domain research scales of Medical-Technology and Feeding PROMs are ready for patient-centered research purposes. Use of these PROMs in clinical practice, (i.e., < 15-20 items), can be achieved with additional participants. More responses will address analytical challenges that are requisites for Rasch models, which can shorten the scales, with greater precision, for use in routine quality-improvement, benchmarking, and testing patient-centered care interventions.