Health Equity/Social Determinants of Health
Session: Health Equity/Social Determinants of Health 3
471 - The application of quality improvement concepts, strategies, and tools to enhance participation in clinical trials among Latino families
Friday, May 3, 2024
5:15 PM - 7:15 PM ET
Poster Number: 471
Publication Number: 471.352
Publication Number: 471.352

Manuel E. Jimenez, MD, MS (he/him/his)
Associate professor
Rutgers, Robert Wood Johnson Medical School
New Brunswick, New Jersey, United States
Presenting Author(s)
Background: Underrepresentation of racial/ethnic minority groups in clinical trials threatens external validity of clinical and translational science, diminishes uptake of innovations into practice, and restricts access to the potential benefits of participation. Despite efforts to increase diversity in clinical trials, children and adults from Latino backgrounds remain underrepresented.
Objective: To describe how we applied quality improvement concepts, strategies, and tools to enhance recruitment and enrollment of Latino caregiver-child dyads from under-resourced communities in a clinical trial testing behavioral interventions designed to promote equity in language and social-emotional skill acquisition for these dyads.
Design/Methods: We enrolled 630 caregivers with children aged 6-11 months from three NJ Community Health Centers (CHCs) engaged in Reach Out and Read (ROR) literacy promotion that serve Latino families from under-resourced communities in an IRB-approved clinical trial. Caregivers needed to be able to provide informed consent, identify as Latino/a/x, have fluency in English or Spanish, be ≥18 years old, have no intent of leaving their CHC, and be willing to be randomized into one of three study arms: (1) ROR; (2) ROR + text messages encouraging reading ; and (3) ROR + text messages + referral to Connecting NJ, a hub for child development, parenting, and community resources. Children needed to be without developmental delays or congenital anomalies or genetic disorders.
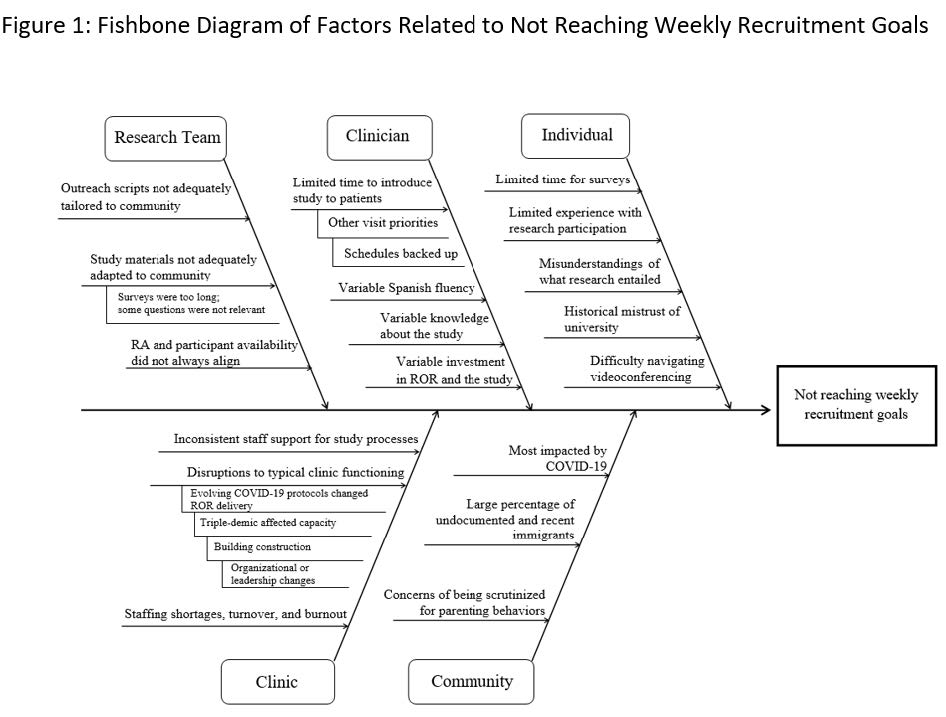
Results: Recruitment activities took place during the COVID-19 pandemic, which intensified the need for responsive procedures. Informed by the Model for Improvement, we engaged strategies such as intentional team formation, participatory approaches to setting goals, establishing measures and selecting changes, and small iterative tests of change and implementation of changes that helped our team overcome several barriers to achieve our recruitment goal (Figure 1). Over the study period, we saw an increase in enrollments eventually exceeding our weekly goals (Figure 2). We provide illustrative case examples that occurred throughout different stages of the study.
Conclusion(s): Using quality improvement concepts was highly practical and helped our team overcome several barriers, even in the context of the COVID-19 pandemic. Other investigators may leverage such approaches to enhance participation in clinical trials and promote equity in clinical translational science.
.jpg)
