Neonatology
Session: Neonatal Infectious Diseases/Immunology 2
615 - Vancomycin Dosing in Neonates and Clinical Outcomes: Are Higher Trough Vancomycin Concentrations Better?
Friday, May 3, 2024
5:15 PM - 7:15 PM ET
Poster Number: 615
Publication Number: 615.364
Publication Number: 615.364

Najla Tabbara, PharmD (she/her/hers)
Perinatal Pharmacist
Mount Sinai Hospital
Toronto, Ontario, Canada
Presenting Author(s)
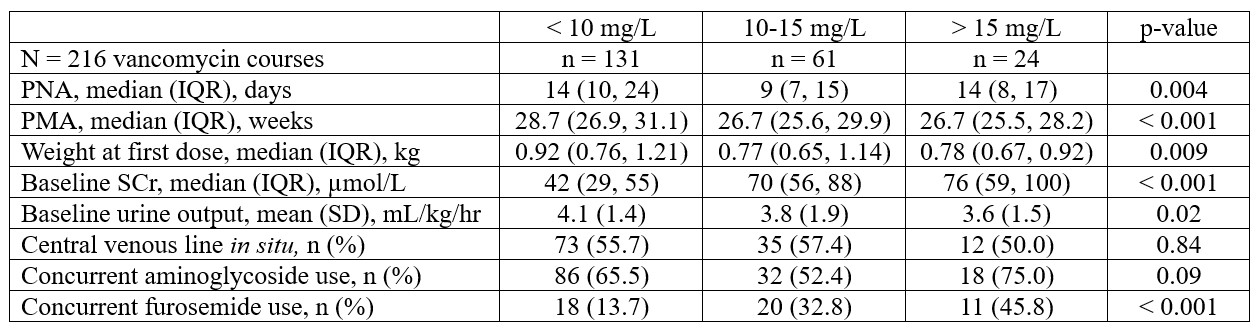
Background: Vancomycin is an antibiotic commonly prescribed for neonatal sepsis and necrotizing enterocolitis. Previous studies suggest that vancomycin concentrations of 10-15 mg/L should be targeted to optimize clinical outcomes. The current empiric vancomycin dosing regimen (10 mg/kg q12h for PMA < 30 weeks and 10 mg/kg q8h for PMA ≥ 30 weeks) was designed to achieve this therapeutic target, however its impact on clinical outcomes is unknown.
Objective: To evaluate the current dosing regimen to achieve initial trough vancomycin concentrations of 10-15 mg/L, and to determine its effectiveness and safety in neonates prescribed ≥ 5 days of therapy.
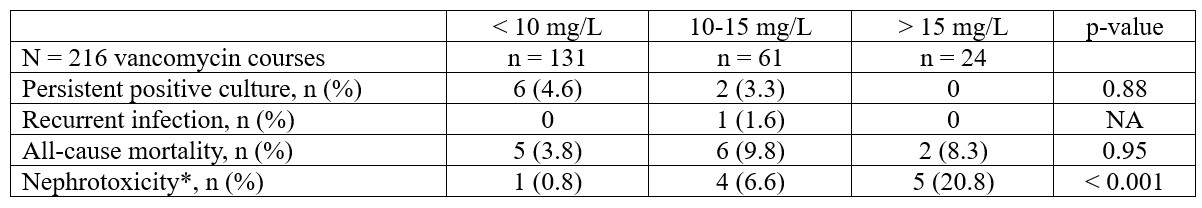
Design/Methods: Retrospective cohort study of neonates admitted to the NICU between October 2016 and December 2021. Neonates were evaluated for: 1) persistent positive culture ≥ 7 days while on vancomycin, 2) recurrent infection within 30 days of vancomycin completion, 3) 30-day all-cause mortality, and 4) acute kidney injury (AKI). A logistic regression model, adjusted for SNAPPE-II and PMA, was used to determine the association between initial vancomycin concentrations, categorized as < 10 mg/L (Group A), 10-15 mg/L (Group B), and > 15 mg/L (Group C), and outcomes. Results are presented as number (%), median (IQR), or mean (SD) as appropriate, and odds ratio (OR) with 95% CI.
Results: Of 521 neonates prescribed vancomycin, 191 neonates with 261 courses had ≥ 5 days of therapy. Baseline characteristics are shown in Table 1. The dosing regimen achieved the vancomycin therapeutic target in only 28% of neonates. Clinical characteristics at vancomycin initiation are shown in Table 2. For the entire cohort, the median (IQR) initial vancomycin level was 8.7 mg/L (6.1, 12.0) and the median (IQR) course duration was 7 days (6, 8). Coagulase negative staphylococci were the most common isolated organisms from blood (79/216; 36.6%), urine (17/158; 10.8%), and cerebrospinal fluid (7/130; 5.4%) cultures. Clinical outcomes are shown in Table 3. Compared to Group A, no difference in the risk of persistent positive culture (OR 0.48, 95% CI 0.08, 2.74) or all-cause mortality (OR 1.85, 95% CI 0.50, 6.83) was noted in Group B. Similarly, no difference in the risk of all-cause mortality (OR 1.49, 95% CI 0.25, 8.75) was noted in Group C vs. Group A. AKI was highest in Group C (20.8%), compared to Group A (0.8%) and Group B (6.6%).
Conclusion(s): Most neonates did not achieve the initial vancomycin concentration target with the current dosing regimen. Vancomycin concentrations >10 mg/L did not improve clinical outcomes but increased AKI risk.
.jpg)