Nephrology
Session: Nephrology 4
22 - Improving RAASi Prescribing in Children with CKD
Sunday, May 5, 2024
3:30 PM - 6:00 PM ET
Poster Number: 22
Publication Number: 22.2016
Publication Number: 22.2016

Rishil Patel, MBBS BSc (he/him/his)
Paediatric Nephrology Registrar
Great Ormond Street Hospital
Childs Hill, England, United Kingdom
Presenting Author(s)
Background: Chronic kidney disease (CKD) is a major health concern worldwide. Renin–angiotensin–aldosterone system inhibitors (RAASi) are anti-hypertensive and anti-proteinuric agents which have been shown to attenuate progression of CKD in children. Failure to prescribe RAASi, in appropriate patients, is a missed opportunity for slowing CKD progression and could have detrimental effects including earlier progression to end-stage kidney disease (ESKD).
Objective: This quality improvement project aimed to increase the percentage of children with CKD stage II-IV, with an indication for a RAASi (clinic BP >/= 90th percentile and/or proteinuria ACR>30 mg/mmol or PCR>50 mg/mmol) who are prescribed RAASi, by 50% over a 9-month assessment period.
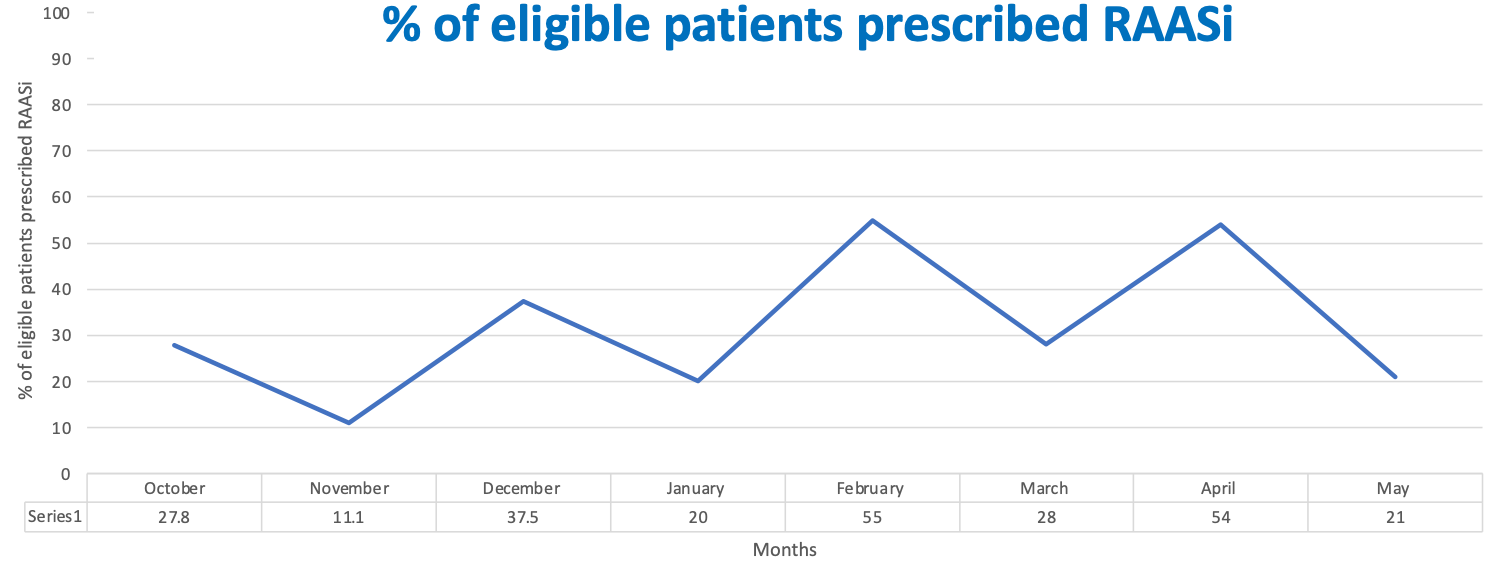
Design/Methods: Baseline data collected in September 2022 indicated 28% of children with CKD were prescribed RAASi. Focus groups and semi-structured interviews with key stakeholders were performed to ascertain the barriers to RAASi prescribing.
Using model for improvement methodology, we designed three Plan-Do-Study-Act (PDSA) cycles; 1) improve awareness of the benefits of RAASi through educational posters and inclusion of a RAASi prescribing prompt in the CKD clinic template to encourage active consideration of RAASi prescribing, 2) increase physician confidence in the accuracy of proteinuria measurement by increasing use of first morning urine samples through phone call and written reminders to patients, and a ‘Two is better than one’ approach: patients given two urine collection cups at each clinic 3) develop a prescribing guideline for RAASi in children with CKD.
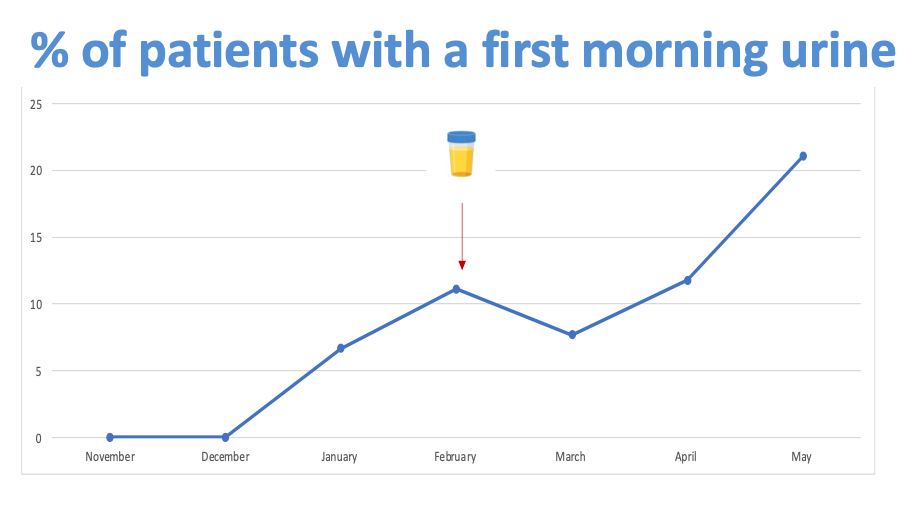
Results: The percentage of children with CKD who were appropriately prescribed a RAASi increased from 22% in October 2022 to 55% in April 2023. PDSA cycle 1 showed good fidelity, with the percentage of children with evidence of active consideration of RAASi prescribing increasing from 20% to 95% post-intervention. PDSA cycle 2 is in early stages of implementation and has shown an increase in patients providing first morning urine samples for proteinuria testing from 0% to 22%. The prescribing guideline is in development and will be implemented as part of PDSA cycle 3.
Conclusion(s): To date this project has resulted in improvement in clinicians considering RAASi prescribing in children with CKD, and increased reliability of the assessment of proteinuria through the use of first morning urine specimens.
.png)
