Neonatology
Session: Neonatal Infectious Diseases/Immunology 1
586 - A Novel TLR2/4 inhibitor (AVR-123) decreases Inflammatory Cytokines in Cord Blood Mononuclear Cells and reduces Cytotoxic Immune Cells in a Murine Model of Retinopathy
Friday, May 3, 2024
5:15 PM - 7:15 PM ET
Poster Number: 586
Publication Number: 586.509
Publication Number: 586.509

Suchismita Acharya, PhD (she/her/hers)
Chief Executive and Chief Scientific Officer
AyuVis Research Inc
Grapevine, Texas, United States
Presenting Author(s)
Background: AVR-123, a small molecule dual inhibitor of Toll-like receptors 2 and 4 (TLR2/4) is in preclinical development for treatment of retinopathy of prematurity (ROP) in premature infants. TLR2/4 overactivation leading to hyper-inflammation with macrophage and neutrophil infiltration are predominant in ROP. We previously demonstrated the VEGF independent anti-angiogenic activity of AVR-123 in an Oxygen Induced Retinopathy (OIR) murine model (Invest. Ophthalmol. Vis. Sci. 2023;64(8):3501)
Objective: To demonstrate the anti-inflammatory activity and downstream signaling of AVR-123 in vitro in cord blood mononuclear cells (CBMCs) and in vivo via change in immune cell populations from the spleen of mouse pups in oxygen induced retinopathy (OIR) model.
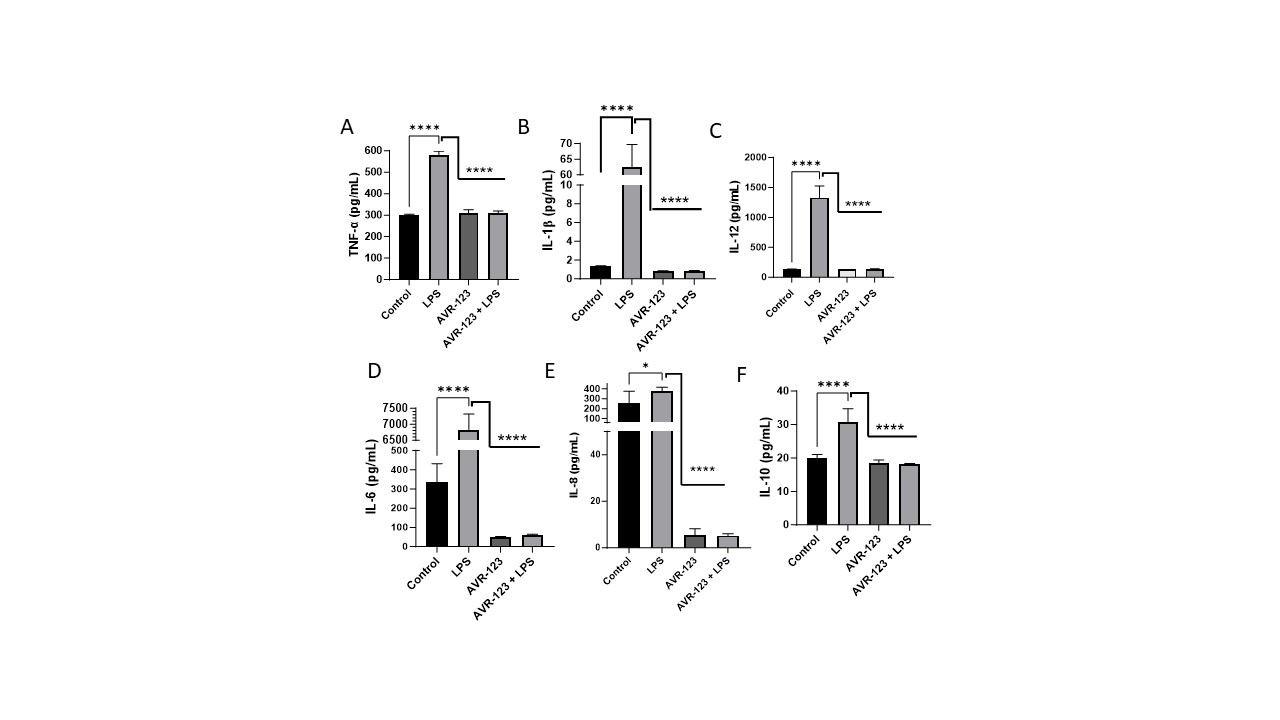
Design/Methods: Cytokine Analysis: CBMC (1.5 million, StemExpress, N=3) were plated in 6-well plates for 4h, then treated with AVR-123 (10, 100 µM), LPS (25ng/mL), and AVR-123+LPS and incubated for 24h. The supernatant was measured for interleukins (IL)-6, IL-8, IL-10, IL-1β, TNF-α, and IL-12p40 levels using ELISA. One-way ANOVA
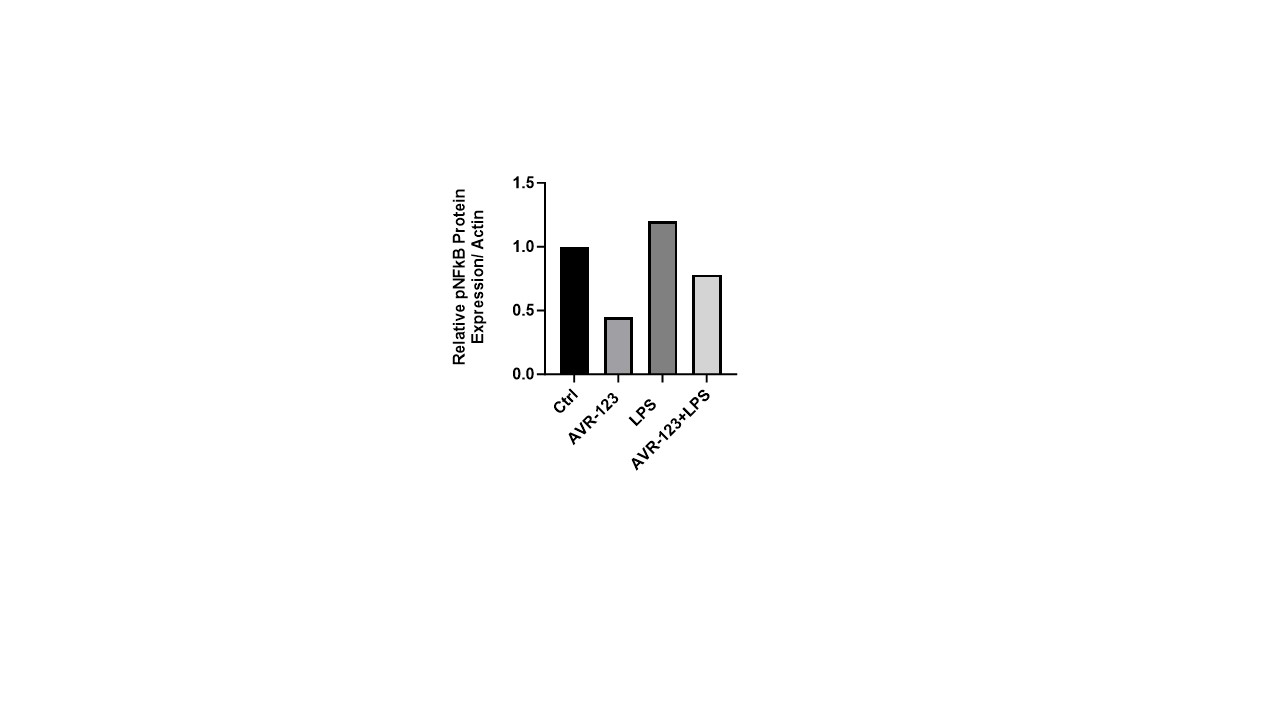
Western Blot: CBMCs (1.5 million, StemExpress) were plated in 6-well plates for 4h, followed by treatment with AVR-123 (100 µM), LPS (50ng/mL), AVR-123+LPS and incubated for 1h. Using western blotting, cell lysates were assessed for downstream TLR2/4 signaling molecules pNF-κB (ABClonal) and ImageJ was used for densitometry.
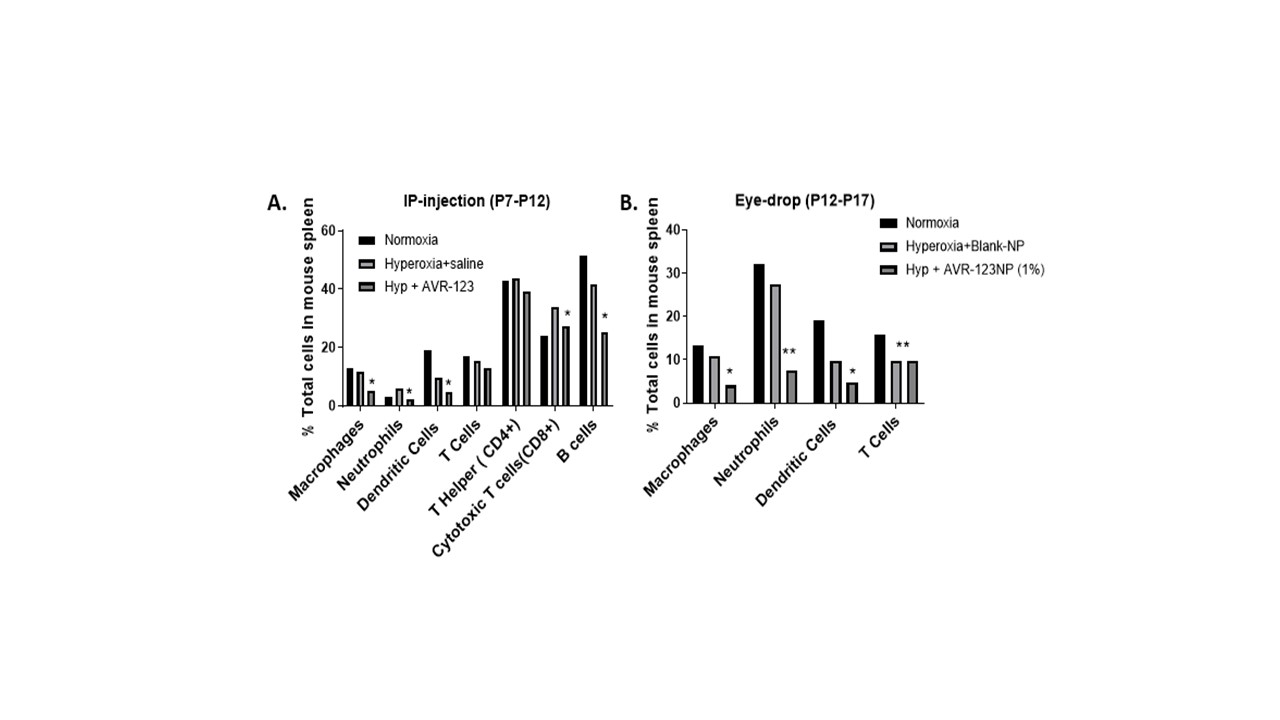
FACS Analysis: Mouse spleens (N=3-5) were collected at P18 from two OIR experiments. 1) Daily intraperitoneal dose of 10mg/kg AVR-123 at hyperoxia (70% O2) phase P7-P12; 2) Daily eye-drop of 1% AVR-123 nanosuspension at hypoxic phase P12-P17. Immune cell surface receptor antibodies were used with appropriate compensation controls to gate immune cell types (FlowJo) and were graphed as percentage populations.
Results: CBMCs treated with AVR-123+LPS show a decrease (~2-4-fold) in cytokine signals in IL-6, IL-8, IL-12, IL-1β, TNF-α and IL-10 indicating anti-inflammatory efficacy as compared to LPS treated cells (Fig. 1A-F).
CBMCs treated with LPS increased phosphorylated NFkB protein whereas AVR-123+LPS decreased the protein level suggesting TLR-2/4 mediated downstream signaling to inhibit inflammatory response (Fig. 2).
In mouse spleen from OIR model, AVR-123 treatment via systemic and topical routes reduced % of macrophages, cytotoxic CD8+T-cells, and neutrophils. (Fig 3)
Conclusion(s): AVR-123 reduces inflammatory cytokines, immune cells and nuclear receptor protein pNFkB related to ROP pathology along with anti-angiogenic effect via TLR2/4 signaling.