Nephrology
Session: Nephrology 2
30 - Treatment and Monitoring of Acute T-cell Mediated Rejection Across Pediatric Kidney Transplant Programs: A Survey Report From the Improving Renal Outcomes Collaborative (IROC)
Saturday, May 4, 2024
3:30 PM - 6:00 PM ET
Poster Number: 30
Publication Number: 30.1184
Publication Number: 30.1184

Rouba Garro, MD
Associate Professor of Pediatrics
Emory University School of Medicine
atlanta, Georgia, United States
Presenting Author(s)
Background: While recent advances in immunosuppression and treatment of rejection resulted in prolonged allograft survival, rejection remains the leading cause of allograft loss in pediatric kidney transplant (KT) recipients. Although current literature supports the use of steroids and/or lymphocyte depleting agents as treatment for Acute T-Cell Mediated Rejection (TCMR), treatment approaches have not been standardized.
Objective: We examined variability in treatment of TCMR and allograft monitoring practices across pediatric KT programs.
Design/Methods: A voluntary web-based survey was distributed in 2023 to pediatric KT programs participating in IROC to evaluate practice patterns in allograft monitoring/surveillance and management of TCMR post-transplant.
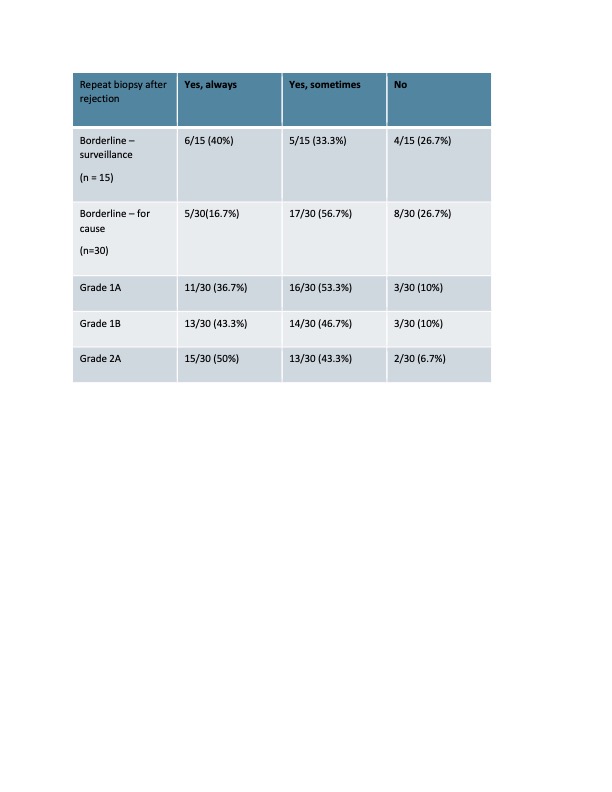
Results: Thirty of 43 (69%) IROC centers completed the survey. Fifteen of 30 (50%) centers performed surveillance biopsies in all or selected patients, with variability in timing of the biopsies. All centers performed other immunologic monitoring post KT, with Donor Specific Antibodies (DSA) being the most common (29/30), followed by donor derived cell free DNA (16/30, 53%). Anti-thymocyte Globulin (ATG) was the most common immunosuppressive medication used for induction therapy (20/30, 66%), with the most common dosing regimen of 1.5 mg/kg/dose for 4 doses (11/30, 36%) followed by 3 doses (9/30, 30%). We assessed treatment practices according to severity of the TCMR as defined by Banff classification. We found 24/30 centers used Methylprednisolone pulse to treat borderline rejection with maximum regimen of 10 mg/kg/dose x 3 doses. 3/30 centers used ATG in addition to steroids to treat Banff 1A TCMR, 28/30 for Banff 1B TCMR, and 30/30 centers used ATG to treat Banff 2A on for cause biopsy. The cumulative dose of ATG used to treat rejection was highly variable across transplant centers ranging from 4 to 9 mg/kg (Figure1). There were also differences in practice regarding monitoring after a rejection episode, including whether a follow up biopsy was performed (Table 1) and the time of the biopsy.
Conclusion(s): There are limited data to guide optimal treatment for TCMR based on Banff grade for pediatric KT recipients. This report highlights the variability in surveillance monitoring and treatment of TCMR post-KT across pediatric centers. Large multicenter pediatric studies using standardized clinal pathways to treat rejection should be considered to evaluate the ideal post-KT monitoring strategy and to determine the effect of different treatment approaches on long-term outcomes in pediatric KT recipients.
.jpg)