Neonatology
Session: Neonatal Nephrology/AKI 1
40 - Perinatal factors associated with neonatal total kidney volume normalized to body surface area
Sunday, May 5, 2024
3:30 PM - 6:00 PM ET
Poster Number: 40
Publication Number: 40.2213
Publication Number: 40.2213
.jpg)
Marissa J. DeFreitas, MD (she/her/hers)
Associate Professor
University of Miami Leonard M. Miller School of Medicine
Weston, Florida, United States
Presenting Author(s)
Background: Programming of nephron mass is impacted by maternal and pregnancy related stressors in experimental models. The clinical association between neonatal kidney size, a known surrogate of nephron mass, and perinatal risk factors is largely unknown. Total kidney volume normalized to body surface area (TKV/BSA) is normally distributed in healthy children aged 0-18 years; however, neonatal data especially in diverse, at risk preterm infant groups are lacking.
Objective: We sought to compare the distribution of TKV/BSA (ml/m2) of our diverse neonatal cohort across the gestational age (GA) categories to a reference population. We also aimed to determine the maternal, placental, and neonatal factors related to lower TKV/BSA.
Design/Methods: This is a retrospective cohort study of 155 neonates from the Infant Kidney Study admitted to the Neonatal Intensive Care Unit at Holtz Children’s Hospital between 2011-2022. Neonatal kidney ultrasounds were obtained and TKV/BSA was calculated using the equation; length x width x depth x (π/6) (ml/m2). Maternal and neonatal clinical and demographic factors were obtained including categorization of hypertensive disorders of pregnancy. Placental weight, presence of placental vascular lesions and chorioamnionitis from pathology reports were collected. Neonates were categorized into reduced TKV/BSA ≤ 25%ile and TKV/BSA >25%ile for subgroup analysis.
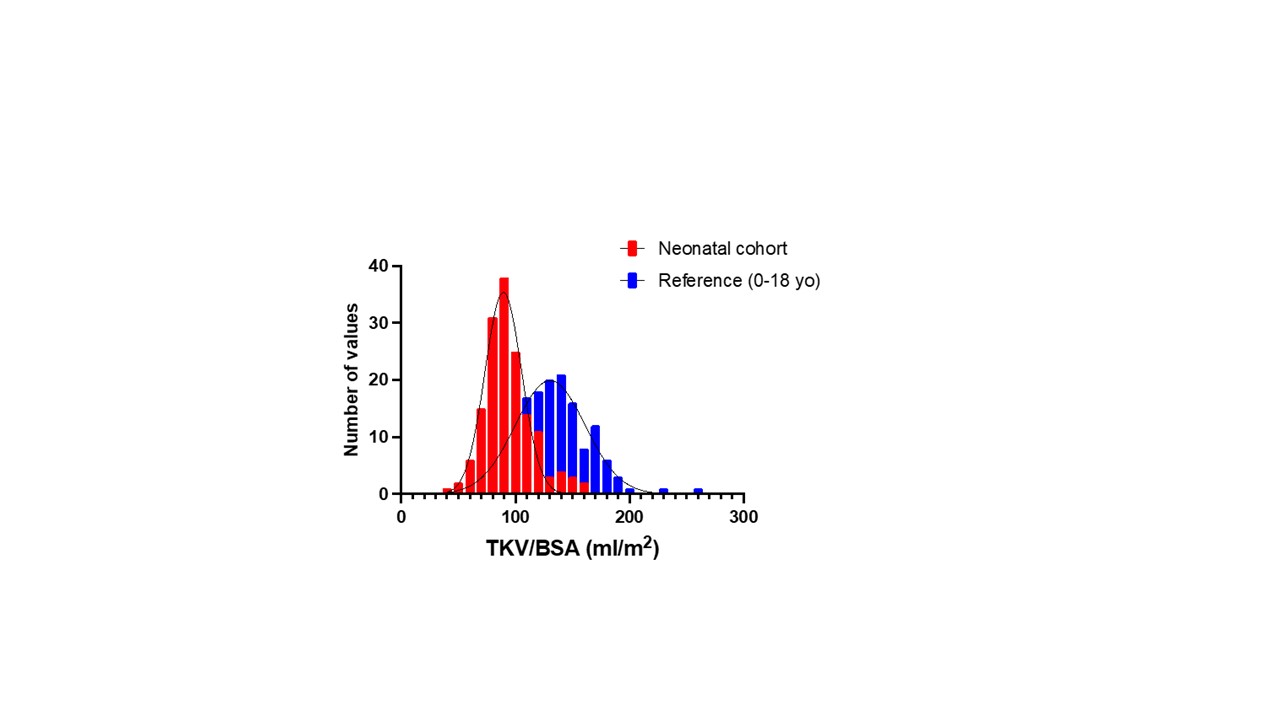
Results: TKV/BSA was lower in our cohort compared to the reference population (91.3 (80.8, 103.4) versus 132 ± 31ml/m2 (Figure 1)). TKV/BSA was higher in preterm (n=90) compared to full term (n=65) neonates (95.2 (83.3, 113.6) ml/m2 versus 87.9 (79.5, 99.0) ml/m2; respectively, p= 0.009). Placental pathology was available in 107 (69%) neonates. Abnormal placental findings were noted in 59/107 (55%). Perinatal factors associated with TKV/BSA percentile group are shown in Table 1. TKV/BSA was significantly higher in neonates exposed to hypertensive disorders of pregnancy than to those not exposed (95.2 (86.3, 120.1) versus 89.8 (80.5, 101.2); p=0.02). TKV/BSA was inversely associated with placental weight (Figure 2).
Conclusion(s): Neonatal TKV/BSA across GA categories was lower in our cohort compared to the reference population. Exposure to maternal hypertension was associated with a larger TKV/BSA percentile. In contrast, higher placental weight, which has been linked to neonatal mortality, was associated with lower TKV/BSA. Further research focused on the role of the placenta in programming of neonatal kidney volume and kidney outcomes is warranted.
.jpg)
.jpg)
