Infectious Diseases
Session: Infectious Diseases 2
64 - Cost-effectiveness of palivizumab for the prevention of severe respiratory syncytial virus (RSV) infection in infants born moderate-to-late preterm: Summary of analyses across 4 continents
Saturday, May 4, 2024
3:30 PM - 6:00 PM ET
Poster Number: 64
Publication Number: 64.1119
Publication Number: 64.1119

Bosco Anthony Paes, MBBS, FRCPC, FRCPI, FAAP (he/him/his)
Professor Emeritus
McMaster University
McMaster University
Dundas, Ontario, Canada
Presenting Author(s)
Background: Palivizumab is effective for preventing severe respiratory syncytial virus infection (RSV) in otherwise healthy infants born moderate-to-late preterm (32-35 weeks’ gestational age [wGA]). Despite its efficacy, the reported cost-effectiveness of palivizumab among 32–35wGA infants varies according to the study and/or country being considered. To support reimbursement decision-making, a new evidence-based cost-utility model (CUA) has been developed that incorporates the International Risk Scoring Tool (IRST).
Objective: Herein we highlight the application of this model by summarizing analyses undertaken in four different continents: North America (Canada); South America (Colombia); Europe (Italy); and Asia (Korea).
Design/Methods: The analysis included 32–35wGA infants identified as being at moderate- or high-risk for RSV-related hospitalization (RSVH) by the IRST in a decision tree model for palivizumab versus no intervention. Infants had RSVH, medically attended (emergency room/outpatient) RSV infection, or were uninfected/non-medically attended, and could experience 6-18 years respiratory morbidity across a lifetime horizon. Palivizumab efficacy was drawn from the IMpact-RSV trial (82.2% RSVH reduction). Country-specific parameters for hospital outcomes, costs, and discounting rates were used. The base case used the healthcare provider perspective with no vial sharing.
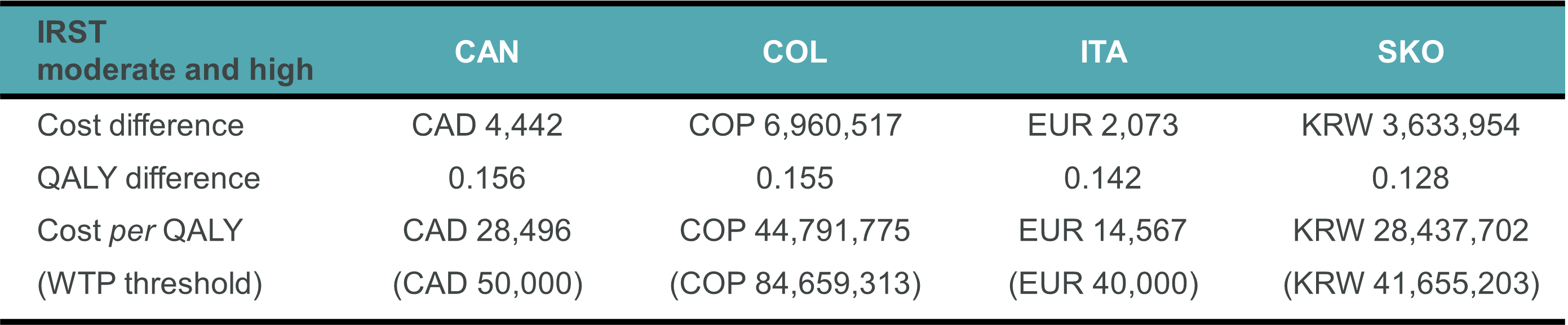
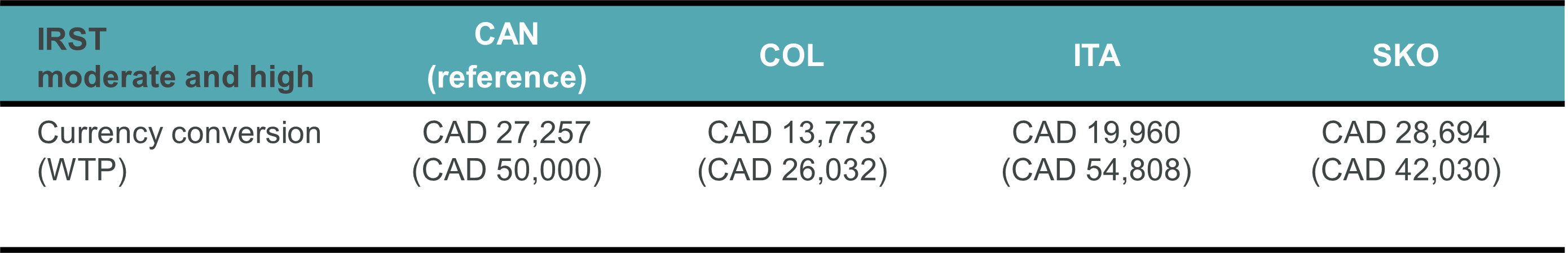
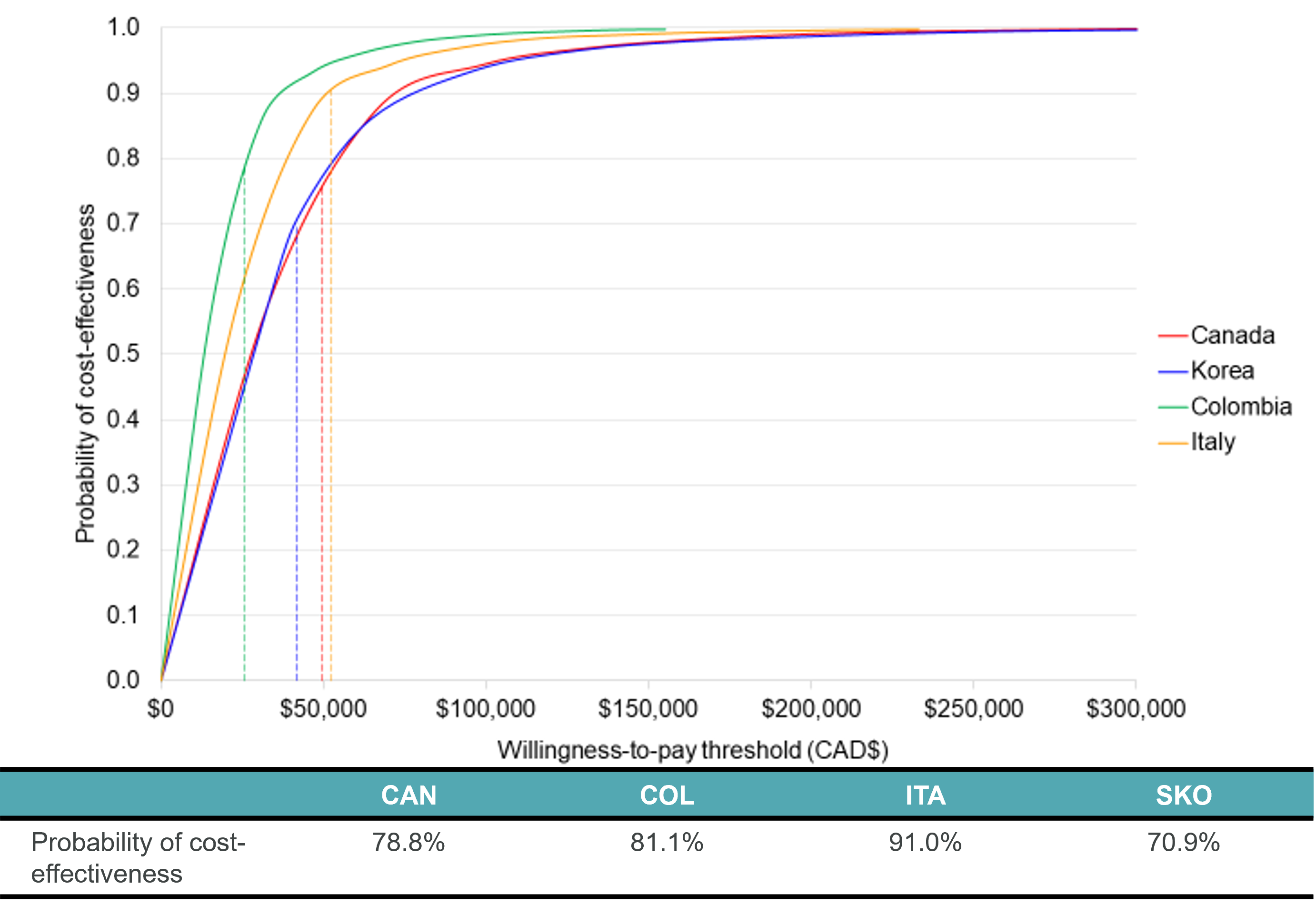
Results: Palivizumab was found to be cost-effective in all countries (Tables 1&2 and Figure 1):
- Canada: CAD 28,496/quality-adjusted life year (QALY) vs willingness to pay threshold [WTP] of CAD 50,000 (78.8% probability of being < WTP)
- Colombia: COP 44,791,775/QALY [CAD 13,773] vs WTP of COP 84,659,313/CAD 26,032 (81.1%)
- Italy: EUR 14,567/QALY [CAD 19,960] vs WTP of EUR 40,000/CAD 54,808 (91.0%)
- Korea: KRW 28,437,702/ QALY [CAD$28,694] vs WTP of KRW 41,655,203/CAD 42,030 (70.9%)
Deterministic sensitivity analysis revealed the key drivers of cost-effectiveness across the models were utility scores, rates of long-term respiratory morbidity in infants who did not receive prophylaxis, and palivizumab efficacy and cost.
Conclusion(s): Risk factor-guided prophylaxis of 32-35wGA infants was a consistently cost-effective strategy versus no prophylaxis across all countries considered. This new CUA has demonstrated utility across different RSV epidemic patterns, geographies and climates, and healthcare systems.