Neonatology
Session: Neonatal Infectious Diseases/Immunology 1
598 - Single Immunoglobulin Interleukin-1 Related Receptor (SIGIRR) inhibits SARS-CoV-2 E protein-induced lung endothelial inflammation and Neonatal lung injury
Friday, May 3, 2024
5:15 PM - 7:15 PM ET
Poster Number: 598
Publication Number: 598.594
Publication Number: 598.594
- HM
Heather Menden, MS
Lab manager
Children's Mercy
Kansas City, Missouri, United States
Presenting Author(s)
Background: SARS-CoV-2 can cause acute lung injury (ALI) in neonates. The envelope (E) protein of SARS-CoV-2 activates Toll Like Receptor 2 (TLR2)-mediated inflammation, endothelial cell (EC) injury and ALI in rodent models. To determine the molecular inhibitors of E protein-induced ALI we focused on SIGIRR, which we have shown to strongly represses TLR signaling in the neonatal gut. We hypothesized that SIGIRR mutations identified in infants with NEC will exaggerate EC inflammation contributing to severe ALI in response to systemic E protein.
Objective: To investigate whether; a) SIGIRR inhibits E protein-induced inflammation and TLR signaling in lung EC and monocytes, and b) neonatal mice encoding the human SIGIRR stop mutation (p.Y168X) have exaggerated ALI after systemic E protein.
Design/Methods: SigirrTg mice encoding the SIGIRR (p.Y168X) mutation were generated using CRISPR-Cas9 gene editing. We developed a neonatal model of E protein-induced ALI using intraperitoneal injections of 2mg/kg in P5 C57BL6 pups. Lung homogenates obtained 48hr after treatments were used to quantify cytokine RNA expression and TLR signaling, while bronchoalveolar lavage (BAL) evaluated inflammatory cell influx. Lung EC (ScienCell) and THP1 cells expressing a NFKB-reporter (Invivogen) were treated with 500ng/mL of E protein after transfecting SIGIRR reference/variant plasmids or SIGIRR shRNA. Cell lysates were used for examining cytokine expression and TLR signaling.
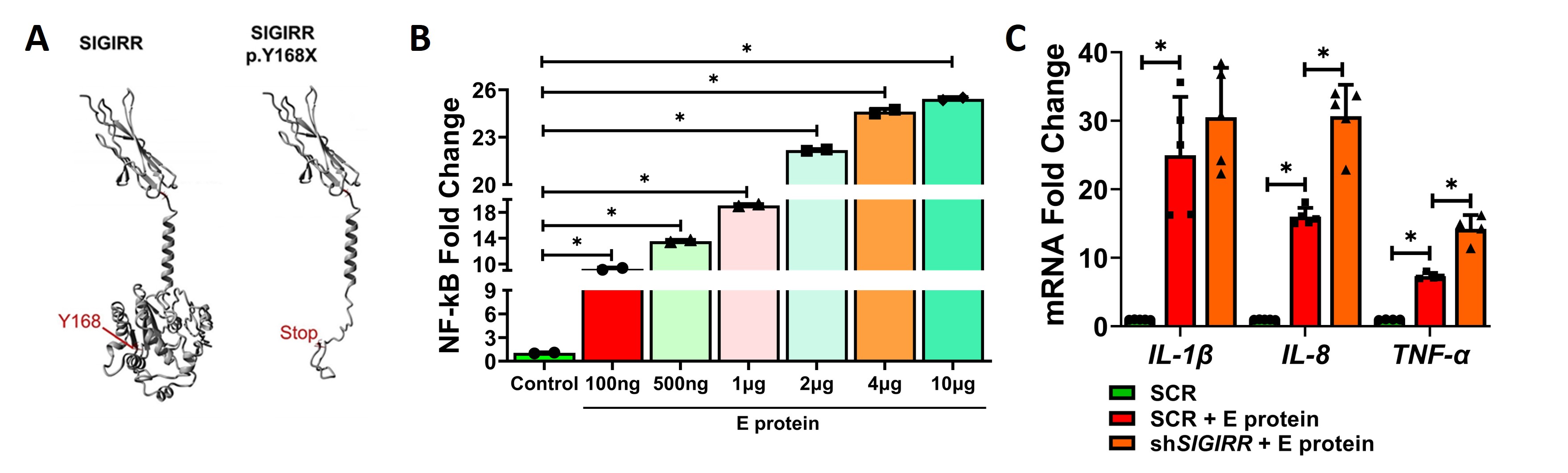
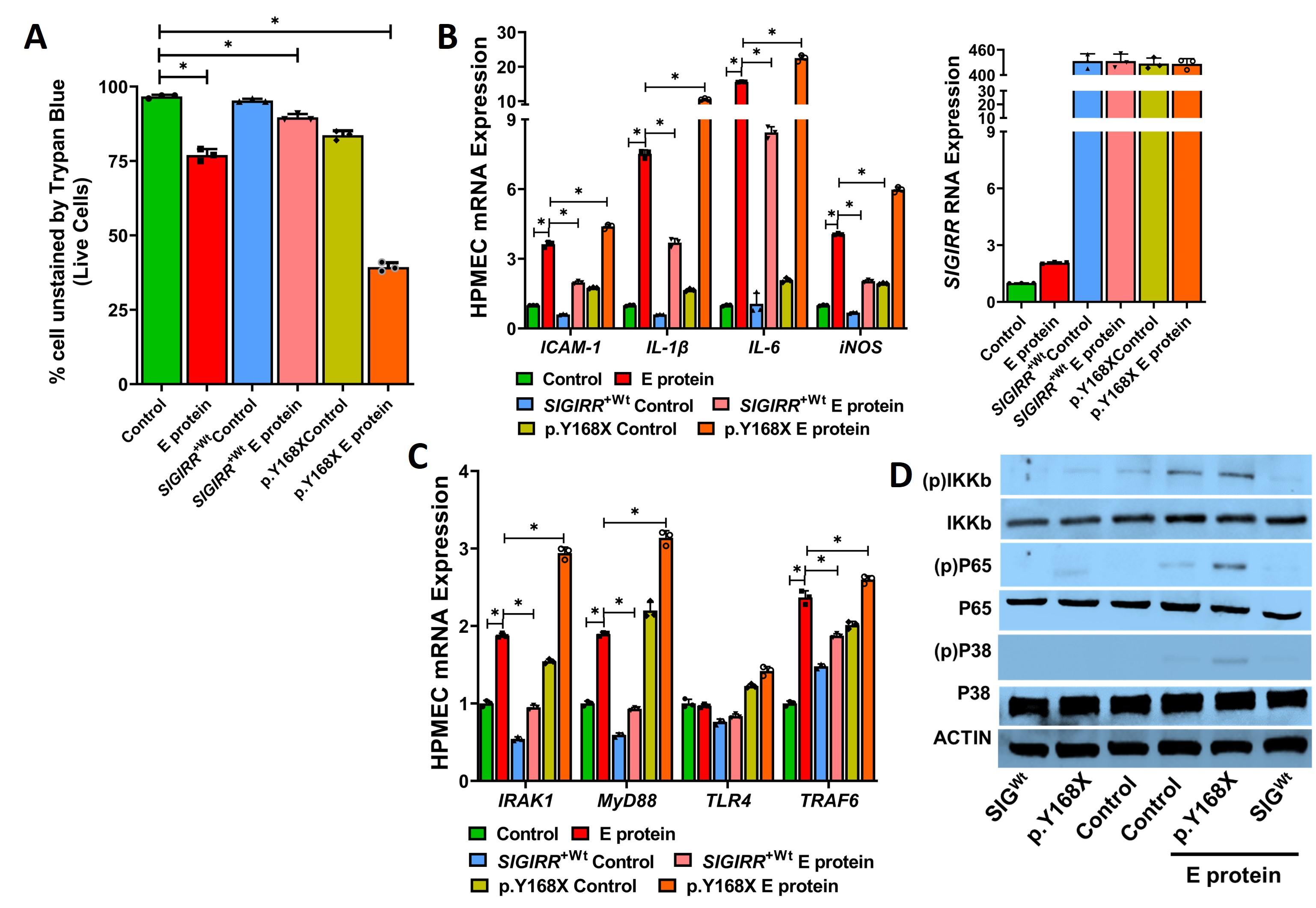
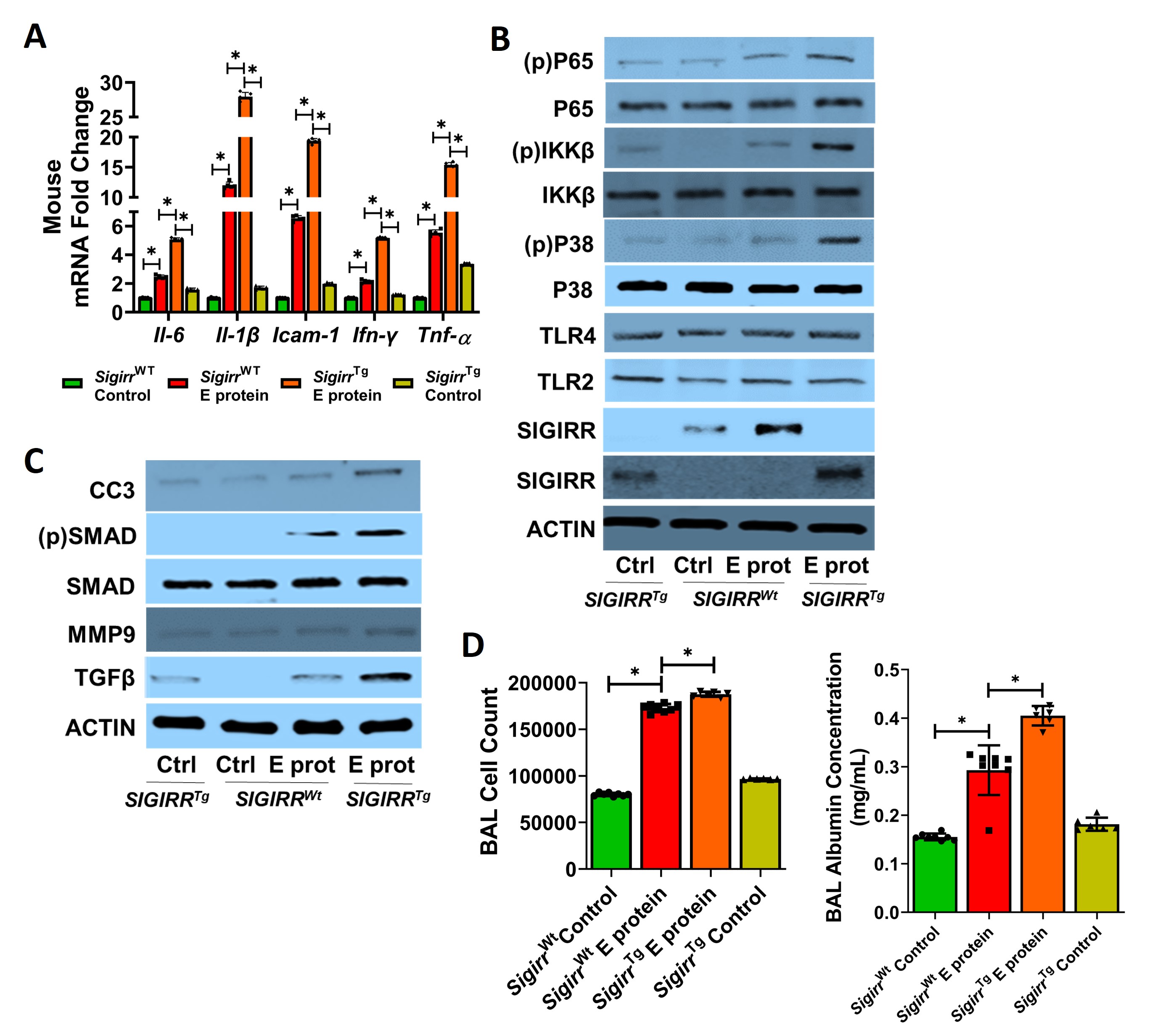
Results: THP1 cells showed a dose-dependent increased in NFKB activity after E protein stimulation. SIGIRR silencing repressed E protein-induced cytokine expression in THP1 (Fig 1). E protein-induced HPMEC death, cytokine expression, and TLR signaling. P65, IKKB, and p38MAPK phosphorylation were inhibited with reference SIGIRR but exaggerated with the p.Y168X SIGIRR variant (Fig 2). The SIGIRR p.Y168X variant induced expression of mediators of TLR signaling including IRAK1, MyD88, and TRAF6.E protein-induced canonical TLR signaling, cytokine expression, and matrix remodeling markers (TGFβ, pSMAD, and MMP9) were amplified in SigirrTg mice. BAL revealed increased inflammatory cellular influx and albumin levels in SigirrTg mice (Fig 3).
Conclusion(s): SARS-CoV-2 E protein-induced EC inflammation, TLR signaling and ALI in the neonatal lung is more severe in mice encoding a loss of function SIGIRR mutation. This study identifies SIGIRR as a key repressor of E protein-mediated EC inflammation and ALI in the neonatal lung.