Neonatology
Session: Neonatal Cardiology and Pulmonary Hypertension 3: Pulmonary Hypertension and Prematurity
107 - Short-Term Outcomes of Invasive Management of Patent Ductus Arteriosus in Very Low Birth Weight Infants in The United States, 2018-2022
Sunday, May 5, 2024
3:30 PM - 6:00 PM ET
Poster Number: 107
Publication Number: 107.1783
Publication Number: 107.1783

Brianna F. Leahy, MBBS
Resident Physician
The University of Vermont Children's Hospital
Burlington, Vermont, United States
Presenting Author(s)
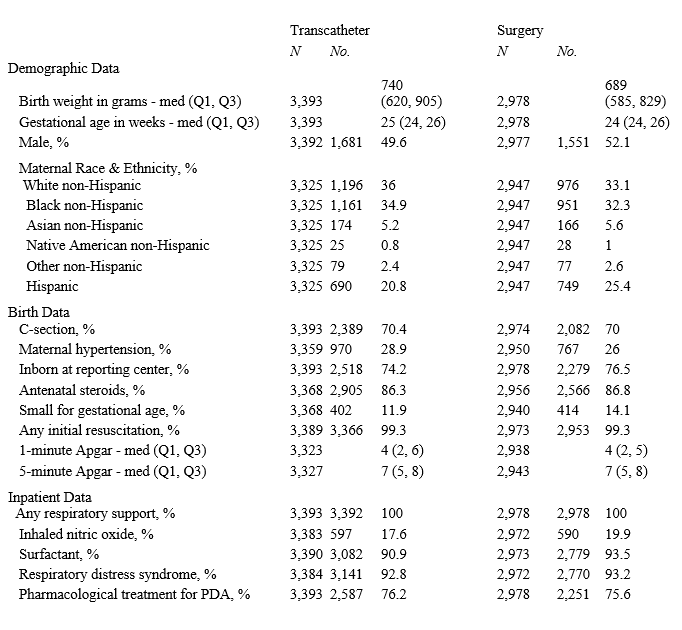
Background: There is variability in the approach to patent ductus arteriosus (PDA) closure in very low birth weight (VLBW) infants. In 2019, the Federal Drug Administration approved the first PDA transcatheter device for preterm infants ≥700 grams (g). The optimal PDA closure method in preterm infants is uncertain.
Objective: To determine survival rates and short-term outcomes for VLBW infants born in the United States (January 1, 2018 - December 31, 2022) who underwent transcatheter PDA closure compared to surgical.
Design/Methods: Secondary analysis of prospectively collected data from Vermont Oxford Network (VON) member hospitals included VLBW infants, defined as 401-1500g birth weight or 22 to 29 weeks’ gestational age (GA), who received invasive PDA treatment, categorized as transcatheter or surgical closure. Infants with congenital heart disease, who did not survive beyond delivery, or had incomplete treatment information were excluded. Data were analyzed by year and averaged over the study duration. Infant characteristics and outcomes with invasive PDA treatment were described using standard summary statistics. Generalized estimating equation regression models were used to compare survival, morbidity, support at discharge and length of stay (LOS) between transcatheter and surgical groups adjusting for GA, small for gestational age, maternal hypertension, inborn/outborn status, and clustering of infants within hospitals. Subgroup analyses were conducted for infants with birth weight ≥ 700g and born in 2020, 2021, or 2022.
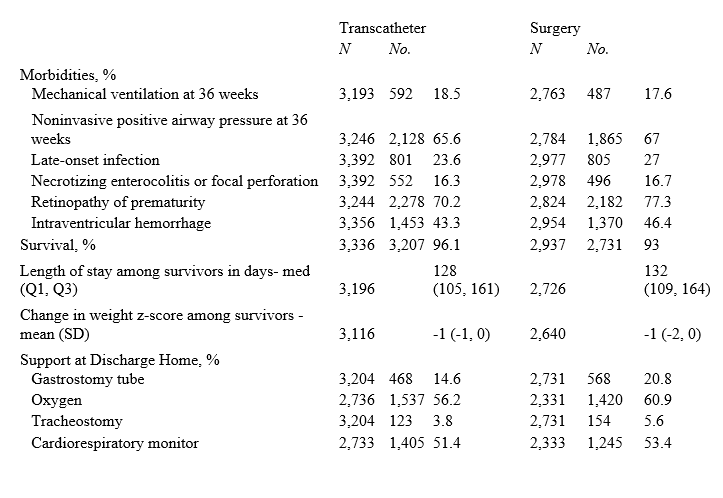
Results: Data from 726 hospitals yielded 216,267 VLBW infants with 41,976 who met inclusion criteria. In infants receiving invasive treatment, transcatheter PDA closure increased from 30% in 2018 to over 70% in 2022 (Figure). Infant characteristics were similar between the two groups (Table 1). VLBW infants who underwent transcatheter closure had higher survival (aRR, 1.03; 1.02, 1.04), and similar morbidity (aRR, 1.00; 0.98, 1.01), support at discharge (aRR, 0.94; 0.89, 1.01) and LOS (aRR, 1.00; -1.32, 3.32), than infants who underwent surgical intervention. In subgroup analyses, survival was similar (aRR, 1.02; 1.00, 1.04) along with support at time of discharge (aRR, 0.90; 0.81,1.01) and LOS (aRR, -4.50; -8.43, -0.58), between the groups, while morbidity was lower (aRR, 0.95; 0.93, 0.98) in the transcatheter group (Table 2).
Conclusion(s): Transcatheter PDA closure in VLBW infants is increasingly utilized since 2018. Short-term outcomes including survival and morbidity following transcatheter closure may be more favorable compared to surgical closure.
.png)
