Neonatology
Session: Neonatal GI Physiology & NEC 3: GI Physiology and Probiotics
543 - Heat Inactivated Probiotics: A Safer Alternative for NEC Prevention?
Sunday, May 5, 2024
3:30 PM - 6:00 PM ET
Poster Number: 543
Publication Number: 543.2249
Publication Number: 543.2249
- CH
Cathy Hammerman, MD (she/her/hers)
Senior neonatologist
Hebrew University Faculty of Medicine
Jerusalem, Yerushalayim, Israel
Presenting Author(s)
Background: Probiotics, live microbial supplements, have been shown to prevent necrotizing enterocolitis (NEC), a devastating disease in preterm neonates. However, safety concerns have been raised regarding the administration of live bacteria to preterm neonates with poorly developed immune defenses and gut maturation. Recently the FDA has issued a general warning voicing these concerns.
Objective: In attempt to minimize the hazards of live bacteria while retaining some of the benefit of probiotic gut protection, we have explored an alternative option using prophylactic heat inactivated probiotic bacteria supplements.
Design/Methods: Randomized prospective study. Preterm neonates < 1500 gm birth weight. Treatment group - daily inactivated probiotic [Biotikid LR, SupHerb [Israel] containing seven types of probiotics, including lactobacillus rhamnosus, lactobacillus acidophilus, lactobacillus caseii, bifidobacteria infantis, bifidobacteria bifidum, bifidobacteria longum, and streptococcus thermophilus] prophylaxis diluted in mother’s milk; and control group - placebo similarly added to milk. All milk suspensions were heated to 80oC for 20 minutes. Probiotic/placebo suspensions were continued until the infant reached 35 weeks post conceptual age.
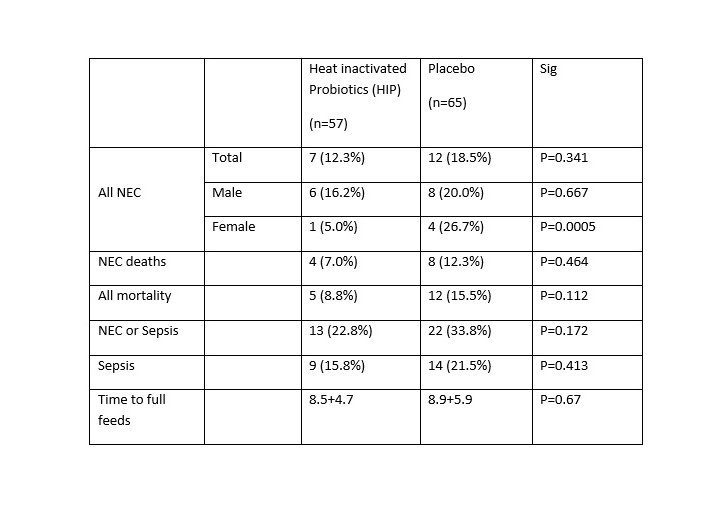
Results: The groups were similar demographically. As seen in table 1, we found no difference in NEC, mortality or sepsis. However, the study was stopped prematurely for logistic recruitment difficulties.
Conclusion(s): We suggest that there may be some protective effect in females in the HIP group which warrants more extensive study.