Neonatology
Session: Neonatal Cardiology and Pulmonary Hypertension 1: PDA
85 - The effect of furosemide on extremely premature infants treated with nonsteroidal anti-inflammatory drugs for persistent patent ductus arteriosus.
Sunday, May 5, 2024
3:30 PM - 6:00 PM ET
Poster Number: 85
Publication Number: 85.2225
Publication Number: 85.2225

Maria del Mar NMN Romero Lopez, MD, MS (she/her/hers)
Assistant Professor of Pediatrics. Division of Neonatology
UT Health Science Center
Houston, Texas, United States
Presenting Author(s)
Background: Persistent Patent Ductus Arteriosus (PDA) is common in infants born at less than 27 weeks gestation. The decision for treatment and what is the best therapy is controversial. Nonsteroidal anti-inflammatory drugs (NSAIDs) are used to close the ductus via the inhibition of prostaglandins. Furosemide can alleviate PDA-induced pulmonary edema but potentially delay PDA closure by increasing renal prostaglandin E2 levels.
Objective: This study aims to evaluate the impact of furosemide administration in infants undergoing NSAID treatment for PDA.
Design/Methods: This retrospective cohort study involved 858 preterm infants born at or before 27 weeks of gestation between 2008 and 2018. We selected the infants who received NSAID treatment for PDA, focusing on severe cases defined as PDA size >2.5mm or PDA size >1.5mm and LA/Ao >2. Demographics, echocardiography, respiratory, hemodynamic, and laboratory data were collected and analyzed based on response to treatment (PDA < 1.5mm) and whether the infant received furosemide between echocardiographic assessments.
Results: A total of 210 infants received NSAIDs (Indomethacin N=124, Ibuprofen N=86). Sixty-four infants met our criteria for severity, and 30 of them received furosemide. No significant differences were observed in basic demographic characteristics regarding furosemide administration. Similarly, there were no notable differences in PDA size, PDA ratio, laboratory values, or fluid intake before NSAID treatment. However, infants who received Furosemide had a higher FiO2 requirement 3 days before NSAID initiation (p=0.027) despite similar mean airway pressure (MAP) (Table 1).
No significant differences were found in PDA closure, post-treatment PDA size, the need for repeat medical treatment, or surgical closure among infants who received Furosemide. Additionally, there were no marked differences in creatinine levels, urinary output, respiratory parameters, or hemodynamic parameters (Table 2).
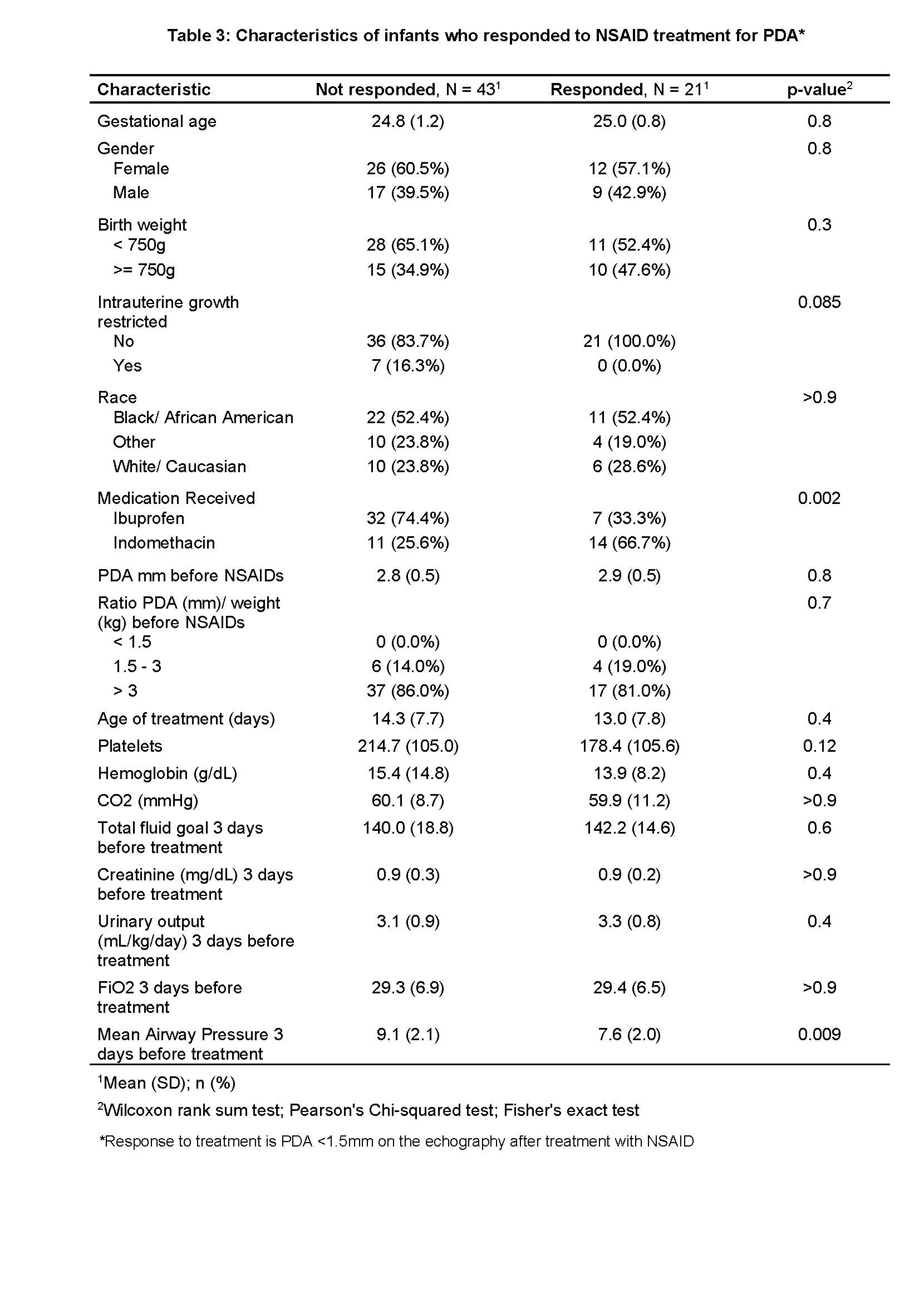
Infants who did not respond to treatment had a higher incidence of intrauterine growth restriction, received Ibuprofen instead of indomethacin, and had a higher MAP before starting NSAID treatment. No significant differences were observed in terms of weight, gestational age, gender, laboratory values, FiO2 pretreatment, or fluid intake during treatment (Table 3).
Conclusion(s): The administration of Furosemide during PDA treatment with NSAIDs does not appear to significantly impact the effectiveness of the therapy. Larger prospective studies are needed to validate and confirm these findings.
.jpg)
.jpg)