Neonatology
Session: Neonatal Neurology 5: Clinical
547 - Predicting Consequences of Intraventricular Hemorrhage in Preterm Neonates using Machine Learning and Targeted Proteomics
Saturday, May 4, 2024
3:30 PM - 6:00 PM ET
Poster Number: 547
Publication Number: 547.1076
Publication Number: 547.1076

Katharina Goeral (she/her/hers)
MD PhD
Medical University of Vienna
Vienna, Wien, Austria
Presenting Author(s)
Background: In recent years targeted proteomics has developed into a powerful protein quantification tool in biomedical research, systems biology, and clinical applications. Preterm neonates with intraventricular hemorrhage (IVH) are at risk for posthemorrhagic ventricular dilatation (PHVD).
Objective: This study aims to inform therapeutic decision-making and parental counseling using proteomics in this high-risk group.
Design/Methods: In this prospective study, we investigated preterm neonates born < 34 weeks of gestation between 2011 to 2023 with intraventricular hemorrhage (IVH).
We performed targeted proteomics analysis on different biological matrices (blood, urine, and CSF), derived from a longitudinal neonatal cohort spanning a decade. We employed explainable machine learning (ML) algorithms to predict PHVD development, patient survival, and identify disease-specific protein-biomarkers. The targeted approach applied in this study was a Proximity Extension Assay (PEA), which combines the specificity of dual antibody recognition with qPCR readout and enables the detection of low concentrations of proteins in biofluids as previously shown in several publications. We applied various ML methods from different domains (statistics, regularization ML, deep learning, decision trees, Bayesian) to the targeted proteomic data for biomarker detection to understand PHVD development and predict the survival of IVH patients. We trained and evaluated 600 models using cross-validation techniques.
Results: A total of 100 patients were included in the analysis, 29 of whom did not survive. Median GA was 25.6 (24.1-27.1), and median IVH grade was 3 (3-4), with bilateral IVH (n=89) in the majority. A significant number of survivors required neurosurgical intervention for PHVD (n=45 of 71 (63%)).
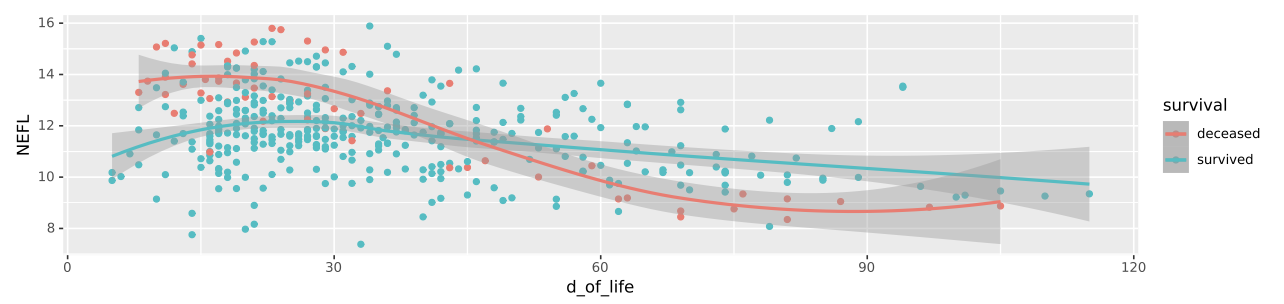
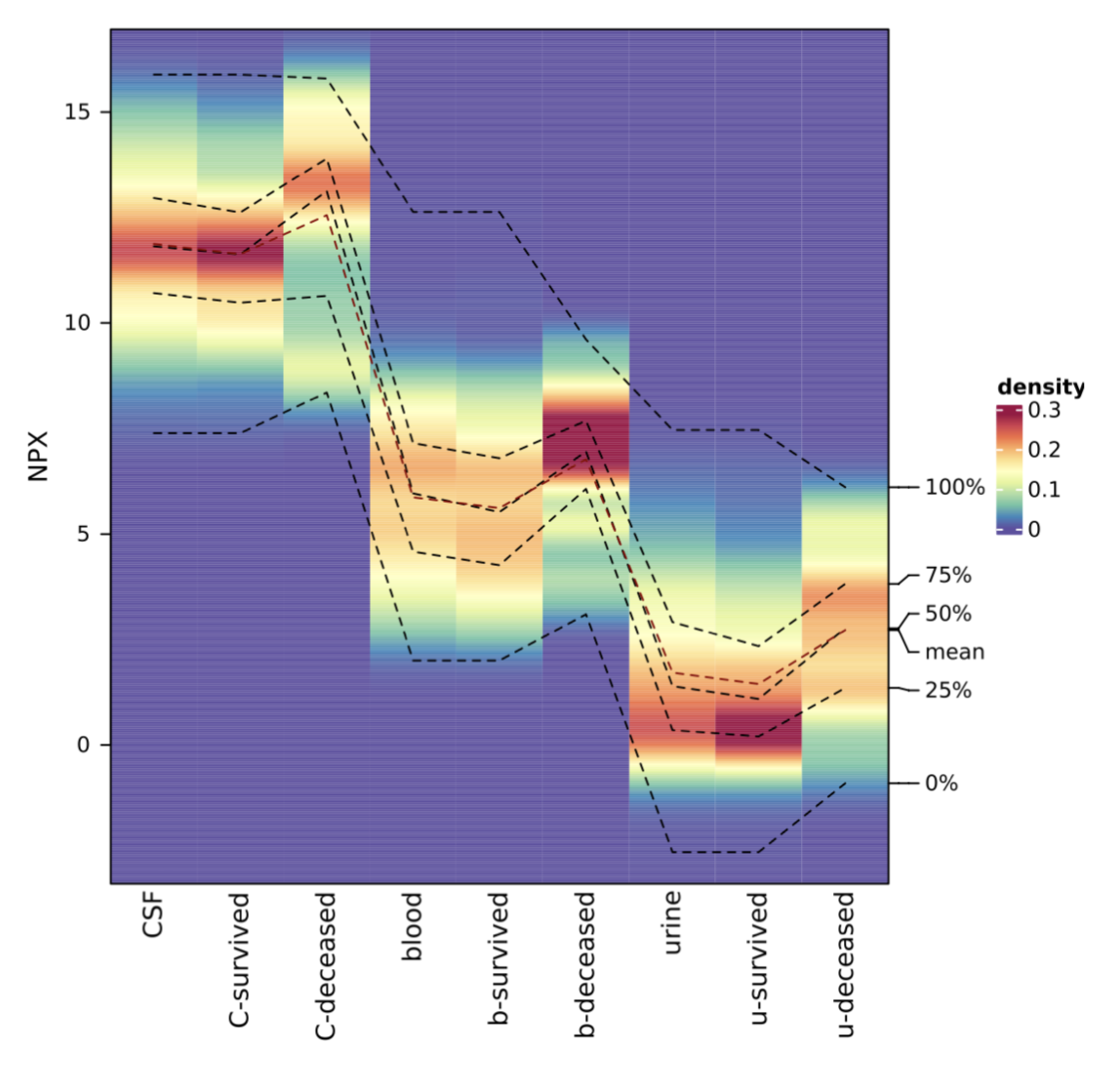
We identified both known (e.g. NEFL (neurofilament light chain)) and novel biomarkers that serve as key indicators for PHVD development and survival prediction. Figure 1 shows a feature plot in CSF of NEFL, while Figure 2 shows NPX values of NEFL in different biofluids between neonates who survived vs. died (distribution shown as heatmap).
Conclusion(s): Our study provides new insights into clinically relevant biomarkers within defined time frames. It demonstrates the robustness of our machine learning models and benefits from a unique neonatal patient cohort.