Neonatology
Session: Neonatal Neurology 9: Preclinical
344 - Using Rat and Ferret Organotypic Brain Slices to Investigate Pathways Associated with Species-Specific Resilience to Injury
Monday, May 6, 2024
9:30 AM - 11:30 AM ET
Poster Number: 344
Publication Number: 344.2922
Publication Number: 344.2922

Olivia C. Brandon, BS (she/her/hers)
Research Scientist
University of Washington School of Medicine
Seattle, Washington, United States
Presenting Author(s)
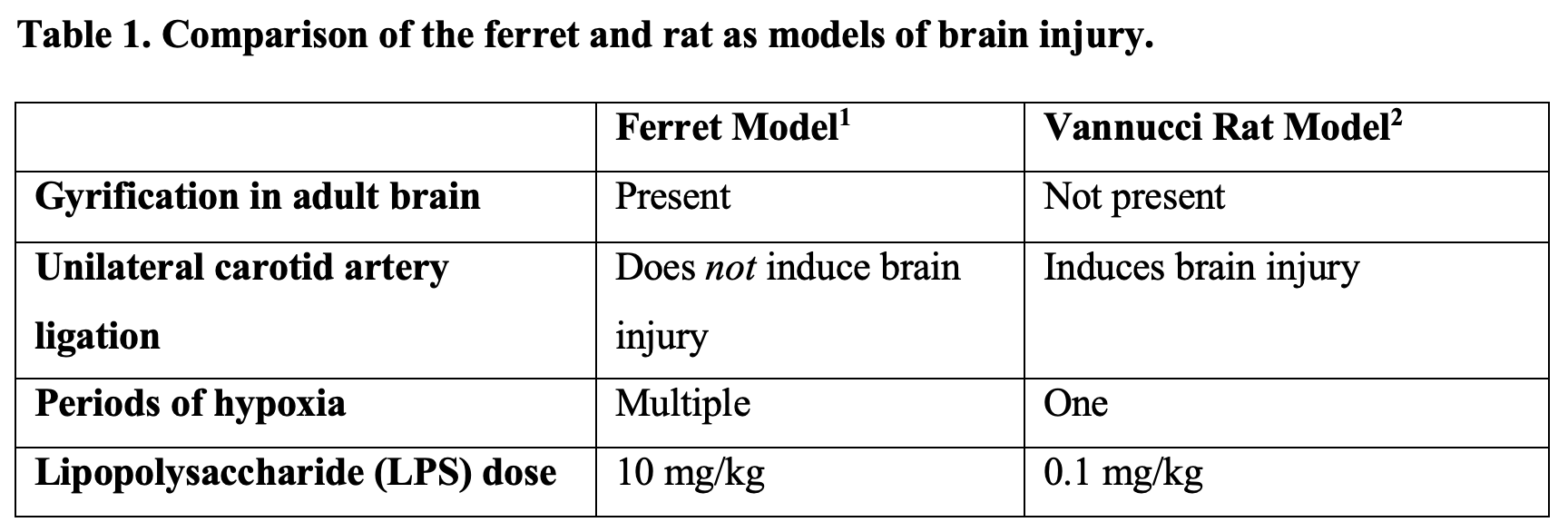
Background: Hypoxic-ischemic injury (HII) is a leading cause of neonatal mortality and morbidity worldwide. The ferret provides an excellent model for investigating treatments for HII as, unlike rodents, it has a gyrified brain and gray-to-white matter ratio that is similar to humans. Previous research has shown the ferret is more resilient to brain injury than the rodent, requiring additional hypoxia periods and greater doses of pro-inflammatory stimuli to create the same injury as in rats (Table 1). However, no previous studies have evaluated why the ferret brain is more resilient than the rodent brain.
Objective: To identify pathways associated with resiliency of the ferret brain to injury at the transcriptome level.
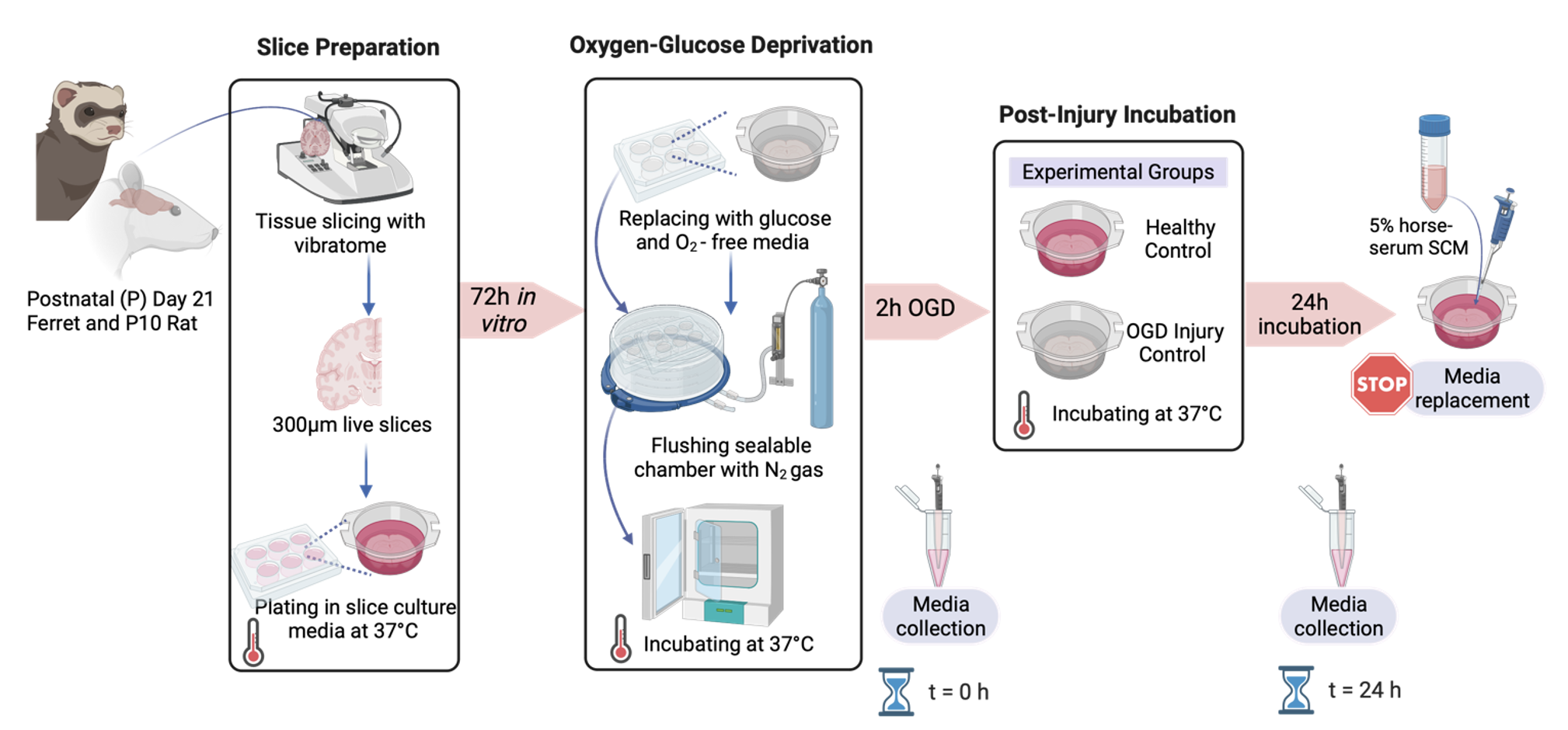
Design/Methods: Whole-hemisphere 300µm brain slices from postnatal day (P)10 rats and P21 ferret, equivalent to term gestation in humans (Fig 1). Slices were randomized to oxygen-glucose deprivation (OGD), to mimic HII, or control groups. OGD slices were exposed to 0% oxygen for 2 hours with glucose-deprived serum. 24h after OGD, global cell death was assessed using lactate dehydrogenase (LDH) release into the culture media. RNA was collected from n=3 slices/sex/species (N=12 slices) and NanoString nCounter digital transcriptomics was performed using a neuropathology panel of 770 genes.
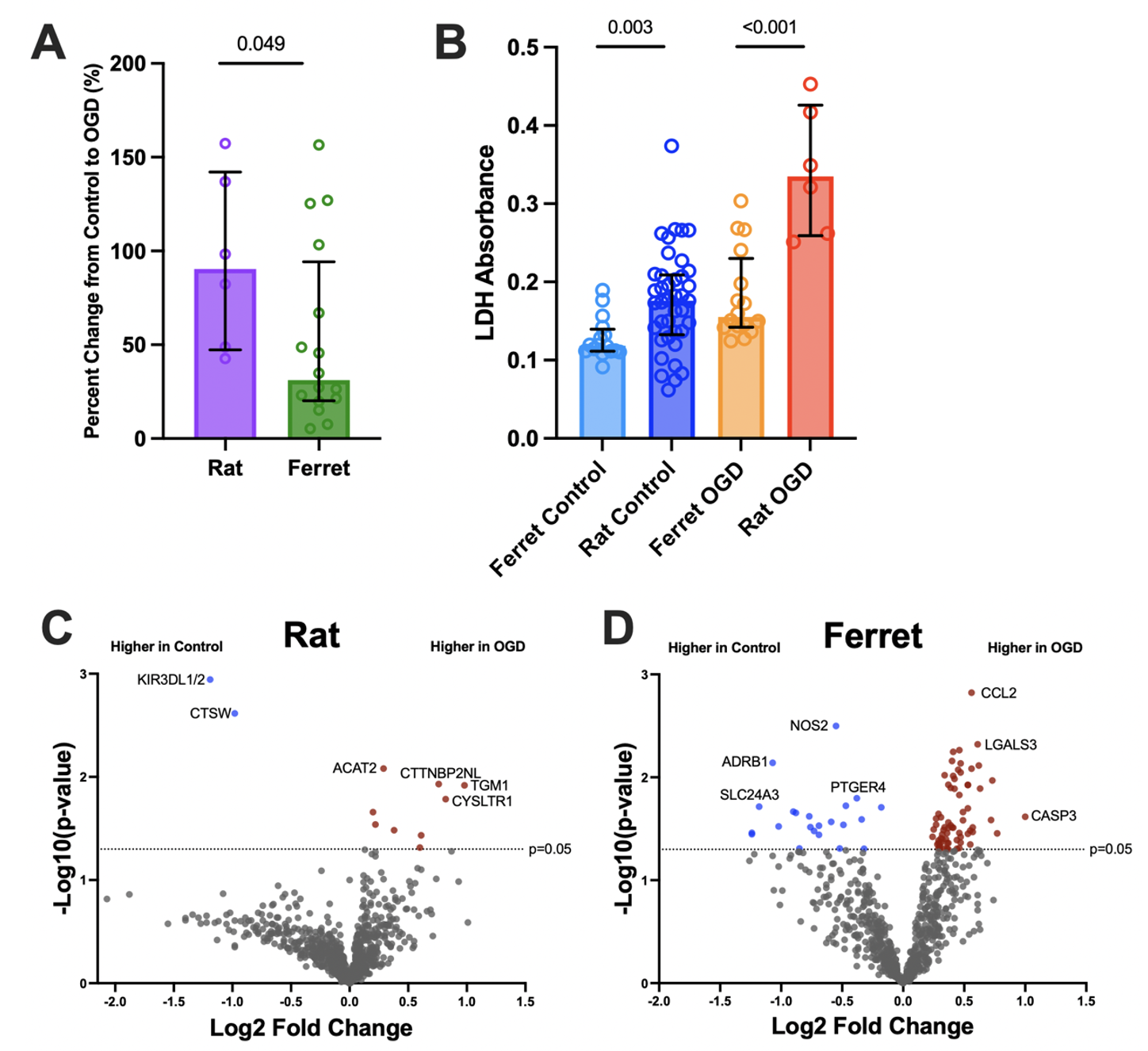
Results: After OGD, rat slices had a significantly higher percent change in LDH release compared to the ferret (p=0.049; Fig 2A) as well as greater LDH release overall (p < 0.001; Fig 2B), signifying greater cell death in the rat with the same OGD exposure. Of the 770 genes assessed, the ferret significantly upregulated or downregulated 90 genes in response to OGD compared to 11 genes in the rat (Fig 2C-D). In response to OGD, the ferret downregulated the expression of ADRB1 (p=0.007) and NOS2 (p=0.003) which are important in regulating adenylyl cyclase and immunity. The ferret upregulated genes including CCL2 (p=0.002) and LGALS3 (p=0.005) that are involved with apoptosis and recruiting potent chemokines. In OGD, the rat downregulated KIR3DL1/2 (p=0.001), a gene involved with natural killer cells and upregulated TGM1 (p=0.01), a gene involved with forming protective barriers.
Conclusion(s): In an established ex vivo organotypic brain slice model of neonatal brain injury, the ferret displayed differential expression of a wider range of genes compared to the rat. Identifying pathways associated with resiliency of the ferret brain to injury at the transcriptome level could inform future therapies to treat infants at risk for HII.