Neonatology
Session: Neonatal Neurology 1: Clinical
13 - Reductions in inter-alpha inhibitor cord blood concentrations in infants with hypoxic-ischemic encephalopathy: Potential biomarkers of neonatal brain health
Friday, May 3, 2024
5:15 PM - 7:15 PM ET
Poster Number: 13
Publication Number: 13.119
Publication Number: 13.119

Lynn Bitar, MD, MSc
Postdoctoral Research Fellow
Division of Neonatal-Perinatal Medicine, Department of Pediatrics, UT Southwestern Medical Center, USA
Dallas, Texas, United States
Presenting Author(s)
Background: Neonates are at risk for infection and inflammation because of their immature immune systems. Inter-alpha inhibitor proteins (IAIPs) are a family of endogenous proteoglycans with a wide range of immunomodulatory effects. Reductions in IAIPs levels have been reported in inflammatory conditions including sepsis and necrotizing enterocolitis. Animal studies have also shown low levels of IAIP in hypoxic ischemic encephalopathy (HIE).
Objective: The focus of this study is to determine blood IAIP concentrations in neonates with HIE.
Design/Methods: This is a single-center prospective cohort study of newborns born at or after 36 weeks of gestation who were admitted to the intensive care unit of Parkland Health hospital over a four years. Blood samples from the umbilical artery (UmA) were collected from neonates before and after placental delivery to measure IAIP levels in three different groups of infants. Term pregnancies were divided into three groups: Group 1 “reference control” included uncomplicated term births delivered by repeat cesarean-section in the absence of labor, group 2 “triple threat” group consisted of women with clinical chorioamnionitis, non-reassuring fetal heart pattern or meconium-stained amniotic fluid with fetal acidosis, whereas group 3, consisted of newborns with moderate or severe hypoxic ischemic encephalopathy (HIE) who met criteria to receive therapeutic hypothermia. Blood levels of IAIPs were quantitatively measured by a competitive ELISA using monoclonal antibodies specifically against human IAIPs.
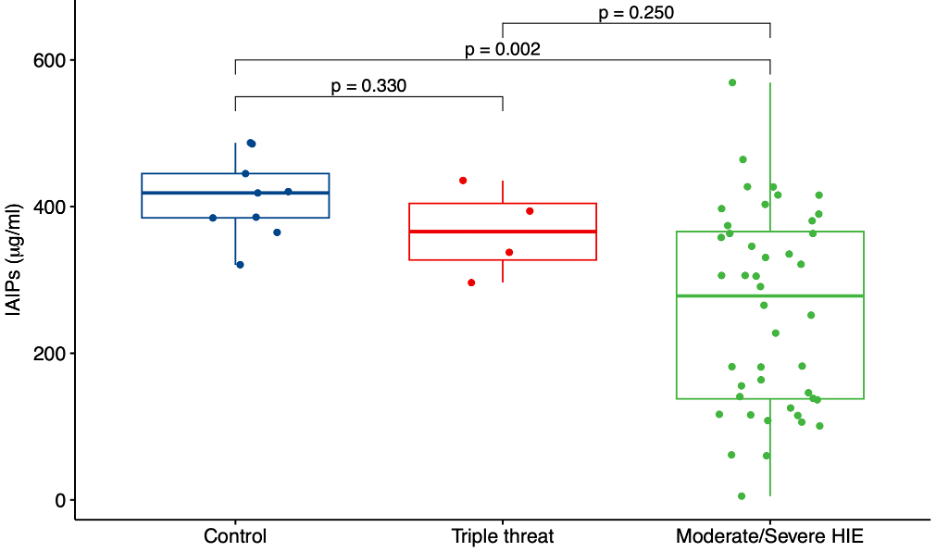
Results: The study included a total of 57 newborns: control (Group 1, n=9), triple threat (Group 2, n=4) and moderate and severe HIE (Group 3, n=44) groups. Measurement of IAIP cord serum concentrations in each of the study groups revealed a significant decrease in IAIP concentrations (p=0.002) in the moderate to severe HIE group compared with each of the other study groups (Figure 1).
Conclusion(s): Infants exposed to moderate to severe HIE have lower concentrations of IAIPs in umbilical arterial cord blood than healthy full-term control infants and infants exposed to a “triple threat". These findings suggest that there is a potential utility of IAIP concentrations in cord blood as biomarkers to determine infants potentially at risk for HIE in neonates. Furthermore, future work could examine the relationship between IAIP concentrations and the severity of HIE. IAIP levels could have diagnostic and therapeutic implications in the management of HIE.