Neonatology
Session: Neonatal-Perinatal Health Care Delivery: Practices and Procedures 3
480 - Effect of Complete Vs. Partial Antenatal Corticosteroid Prophylaxis on Outcomes in Neonates <30 weeks birth gestational age: A Single Center Retrospective Study
Monday, May 6, 2024
9:30 AM - 11:30 AM ET
Poster Number: 480
Publication Number: 480.2907
Publication Number: 480.2907

Christina R. Miller, MD
Resident Physician
University of Tennessee Health Science Center College of Medicine
Memphis, Tennessee, United States
Presenting Author(s)
Background: A single course of antenatal corticosteroids (ACS) is recommended for pregnant women between 24 0/7 weeks and 33 6/7 weeks of gestation who are at risk of preterm delivery. The optimal window for delivery is between 48 hours-7 days after the initial ACS dose. Understanding the effects of varying levels of ACS exposure could prevent or mitigate major preterm birth complications.
Objective: The objective of this study is to assess the relationship between no ACS and varying levels of ACS exposure on neonatal mortality and important morbidities.
Design/Methods: We conducted a retrospective chart review of all infants between 23 and 0/7 weeks and 29 and 6/7 weeks admitted to our level-III NICU from March 2019 to March 2023. Exclusion criteria were delivery room deaths, major congenital and chromosomal abnormalities. Infants were divided into four groups based on betamethasone exposure: no ACS, 1 dose and delivery within 24 hours of initial dose, 2 doses and delivery within 48 hours of initial dose, and complete i.e. 2 doses and delivery between 48 hours to 7 days after initial dose.
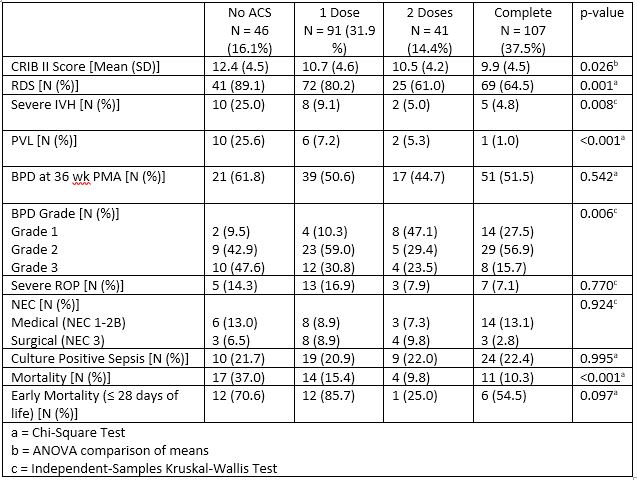
Results: 285 neonates were included with mean GA 26.3 weeks and mean BW 867 grams. Based on ACS exposure, 46 (16.1%) had none, 91 (31.9%) had 1 dose, 41 (14.4%) had 2 doses and 107 (37.5%) had complete ACS exposure. Characteristics among groups were comparable, except for a difference in rates of cesarean delivery and 5-minute Apgar score (Table 1).
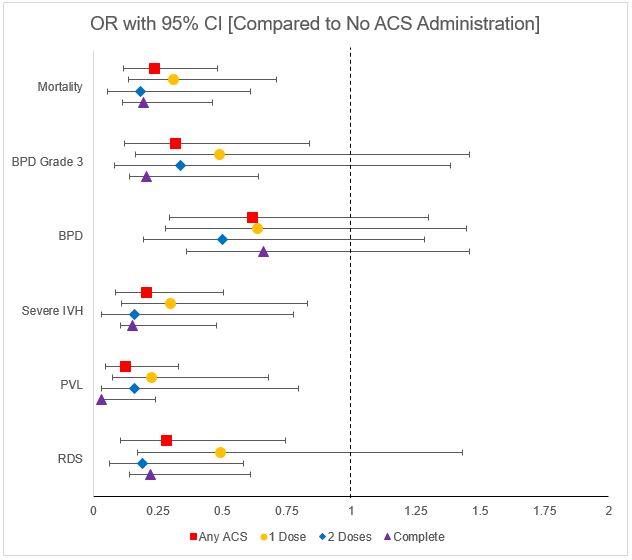
Neonatal outcomes compared between the varying ACS exposure groups are show in Table 2. There is a significant improvement in mean CRIB II score, RDS, severe IVH, PVL, mortality and grade 3 BPD between increasing levels of ACS exposure. Additionally, each level of ACS exposure was compared to no ACS exposure and effect on outcomes was expressed as odds ratio (OR) with 95% CI shown in Fig 1. Any ACS exposure was associated with significantly lower mortality, BPD grade 3, severe IVH, PVL and RDS with complete ACS group having lowest OR of these outcomes.
Conclusion(s): This single center retrospective study shows that there is an association between antenatal corticosteroid administration and reduced risk of neonatal mortality and morbidities such as RDS, severe IVH, PVL and grade 3 BPD as well as the CRIB II score. These reductions were most strongly associated with complete ACS exposure, but a single dose also had benefit. Future adjusted analysis will be performed to adjust for covariates and confounders to better understand these associations.
.jpg)