Neonatology
Session: Neonatology Pulmonology Clinical Science 1: Bronchopulmonary Dysplasia
581 - Mitochondrial DNA Variations May Modulate Neonatal Pulmonary Oxidative Stress and Inflammation
Saturday, May 4, 2024
3:30 PM - 6:00 PM ET
Poster Number: 581
Publication Number: 581.1187
Publication Number: 581.1187
- JK
Jegen Kandasamy, MD (he/him/his)
Assistant Professor
University of Alabama School of Medicine
Birmingham, Alabama, United States
Presenting Author(s)
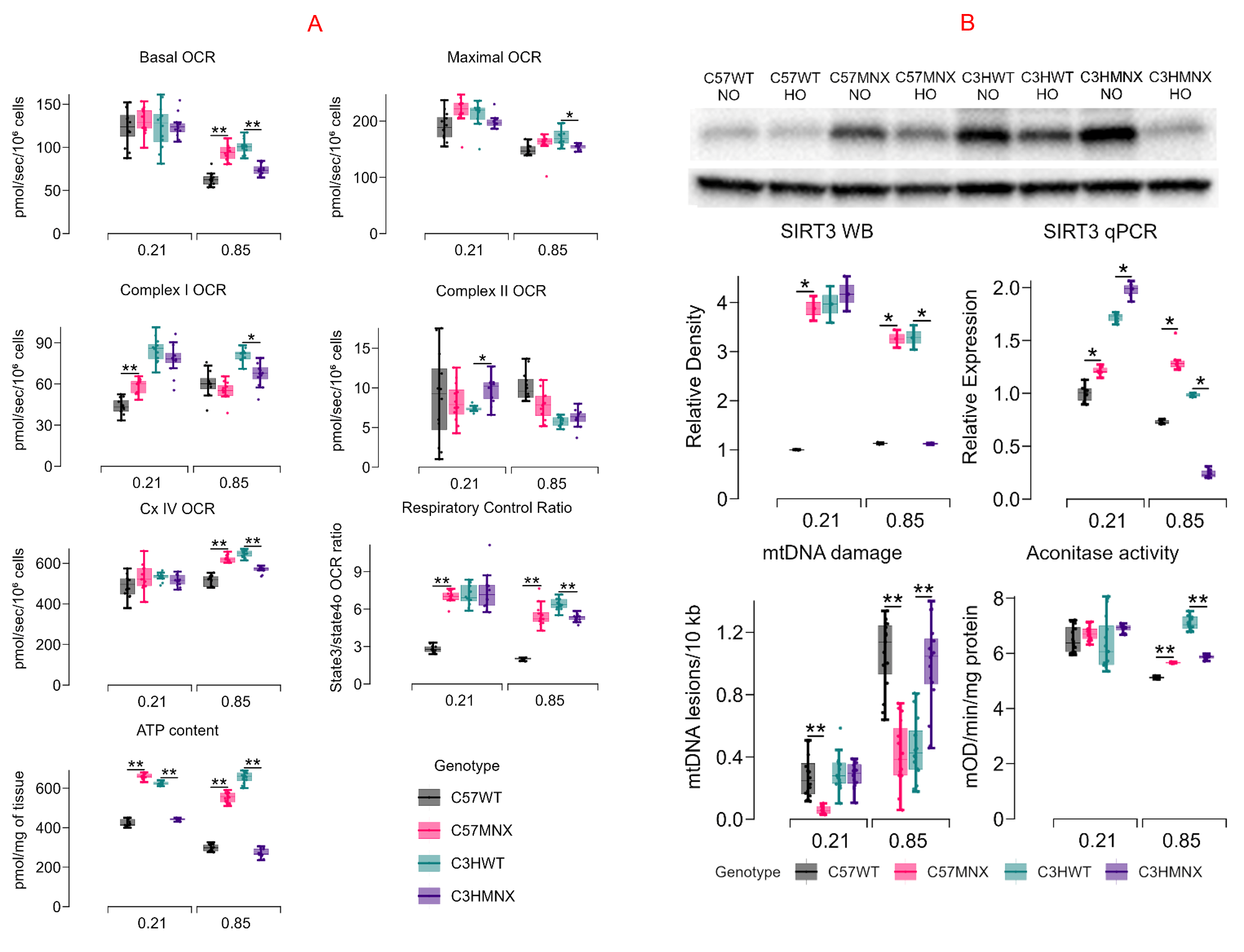
Background: Bronchopulmonary dysplasia (BPD) characterized by inhibition of lung development, inflammation, and fibrosis is the commonest morbidity in very preterm infants. Mitochondrial dysfunction in human umbilical venous endothelial cells (HUVEC) isolated at birth from preterm infants is a strong predictor of BPD. Mitochondrial DNA (mtDNA) differences modulate hyperoxia-induced lung injury severity in neonatal mice, with mice carrying C57 mtDNA (C57 wildtype - WT, and C3H MNX - mitochondrial nuclear exchange) developing more injury vs. mice with C3H mtDNA (C57MNX and C3HWT).
Objective: In this study, we analyzed the effects of mtDNA variations on mitochondrial function, oxidative and inflammatory stress, and quality control (mitophagy).
Design/Methods: We measured ATP generation and the expression of mitophagy (PINK1, Parkin) and oxidative stress related (SIRT3) proteins in HUVEC and tracheal aspirates from Black (mtDNA haplogroup H) and White (mtDNA haplogroup L) infants who subsequently developed BPD. We also exposed newborn WT and MNX mice to hyperoxia and assessed bioenergetic function and oxidant stress in type 2 alveolar epithelial cells (AT2) from their lungs. Lung samples were also analyzed for pro-inflammatory cytokines and transcriptomic profiles. We also evaluated mitophagy in AT2 cells and neonatal mice lung fibroblasts (NMLF).
Results: Our findings revealed several significant observations. Firstly, HUVEC and tracheal aspirates from Black infants (mtDNA haplogroup H) displayed reduced mitochondrial ATP generation and lower expression of essential proteins such as SIRT3 (an antioxidant enzyme) as well as PINK1 and Parkin (related to mitophagy) compared to White infants (mtDNA haplogroup L). Additionally, AT2 cells from mice with C57 mtDNA (C57WT and C3HMNX strains) exposed to hyperoxia showed compromised bioenergetic function and increased oxidant stress compared to those with C3H mtDNA. Furthermore, the lungs of hyperoxia-exposed mice carrying C57 mtDNA exhibited elevated levels of pro-inflammatory cytokines compared to those with C3H mtDNA. Notably, distinct KEGG pathways related to inflammation, PPAR/glutamatergic signaling, and mitophagy were enriched in lungs of mice with different mito-nuclear combinations. Finally, mitophagy was reduced by hyperoxia in all mice strains, with a more pronounced effect in AT2 cells and neonatal mice lung fibroblasts from those with C57 mtDNA compared to C3H mtDNA.
Conclusion(s): Our results indicate that mtDNA variations and mito-nuclear interactions may modify oxidant stress responses and neonatal lung injury severity and require further investigation.
.png)
.png)
