Neonatology
Session: Neonatal Pulmonology - Clinical Science 6: Respiratory/Neuro Outcomes, Steroids
581 - Dexamethasone impact on respiratory status and heart rate variability in preterm very low birth weight infants
Monday, May 6, 2024
9:30 AM - 11:30 AM ET
Poster Number: 581
Publication Number: 581.3252
Publication Number: 581.3252

Kelly A. Denhard, BS (she/her/hers)
Medical Student
University of Virginia School of Medicine
Charlottesville, Virginia, United States
Presenting Author(s)
Background: Dexamethasone improves respiratory status in some, but not all, preterm infants. We previously validated a respiratory acuity score (RAS; PMID 30374050) that predicts adverse respiratory outcomes in infants in the Neonatal Intensive Care Unit (NICU). We also previously found that dexamethasone, via its anti-inflammatory effect, improves heart rate variability, reflected by a decline in a heart rate characteristics index (HRCi; PMID 29286932).
Objective: To test the hypothesis that the impact of dexamethasone on respiratory status would correlate with its effect on HRCi.
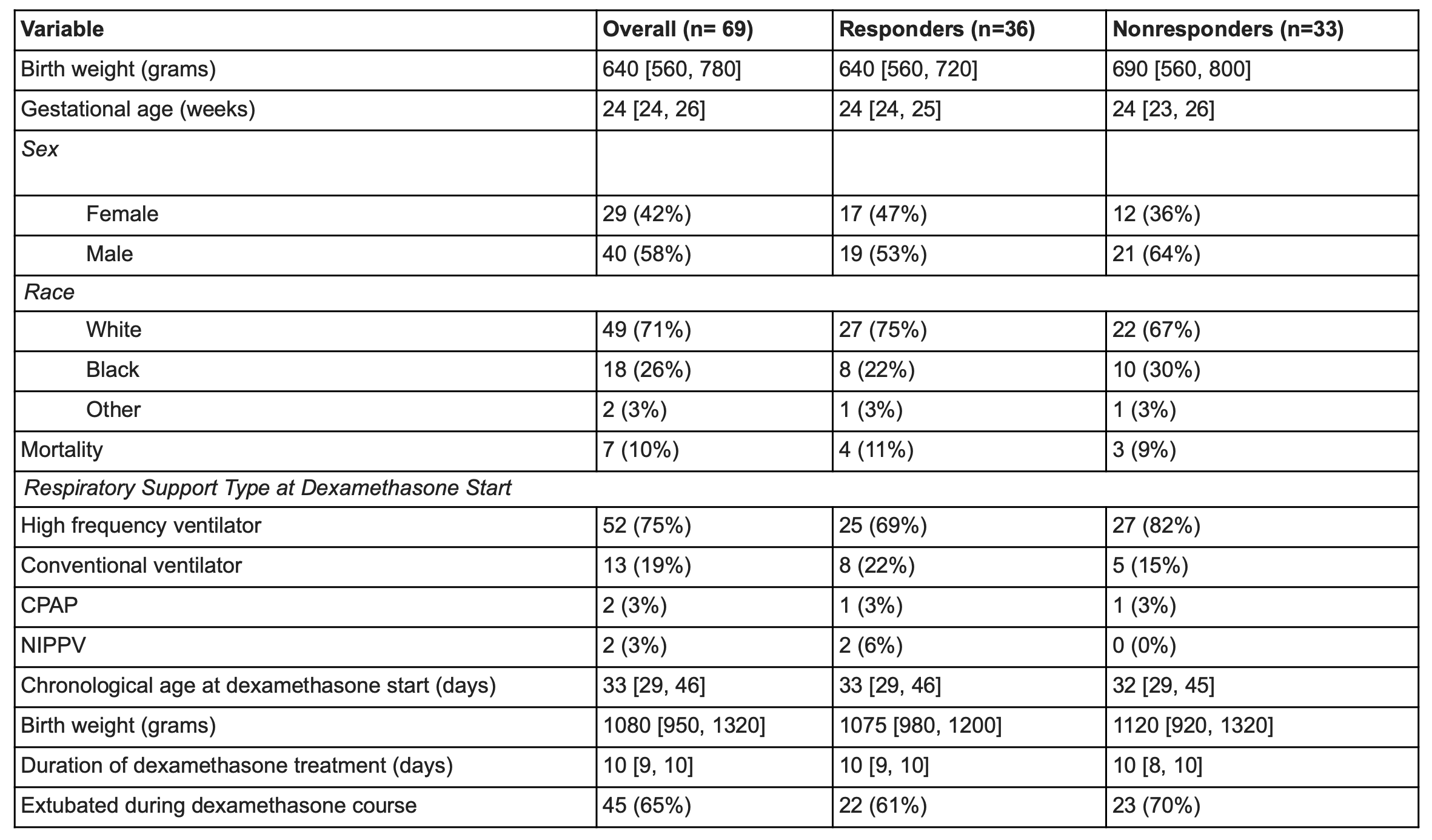
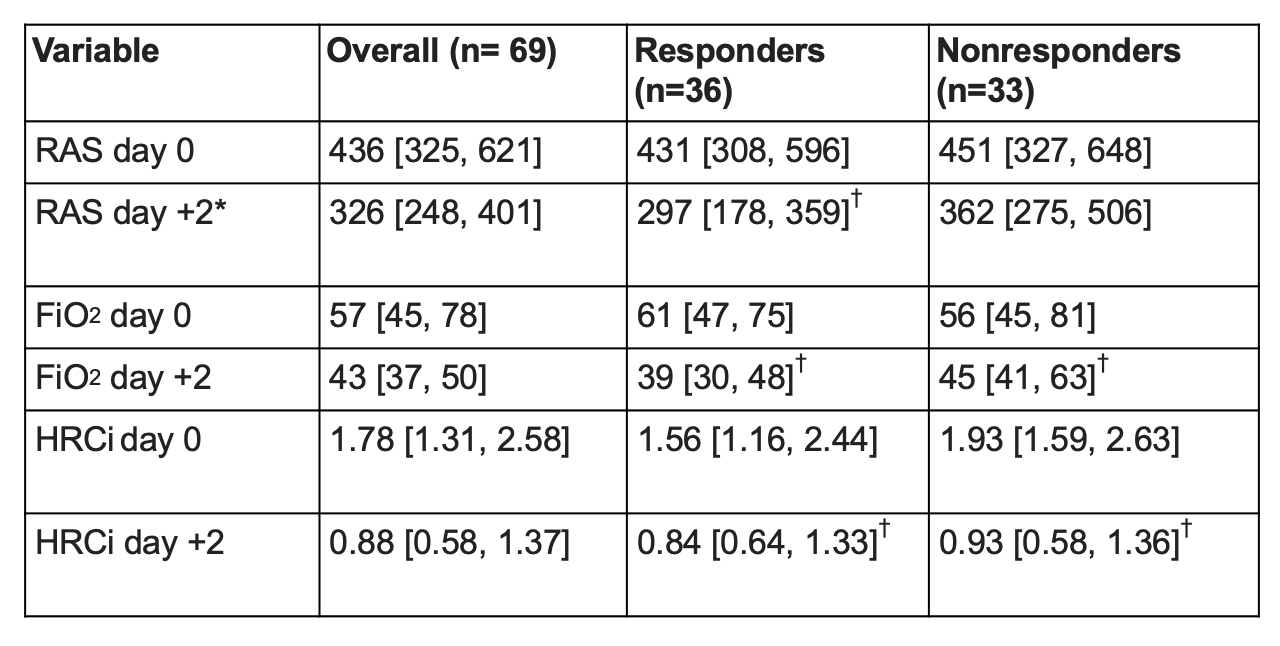
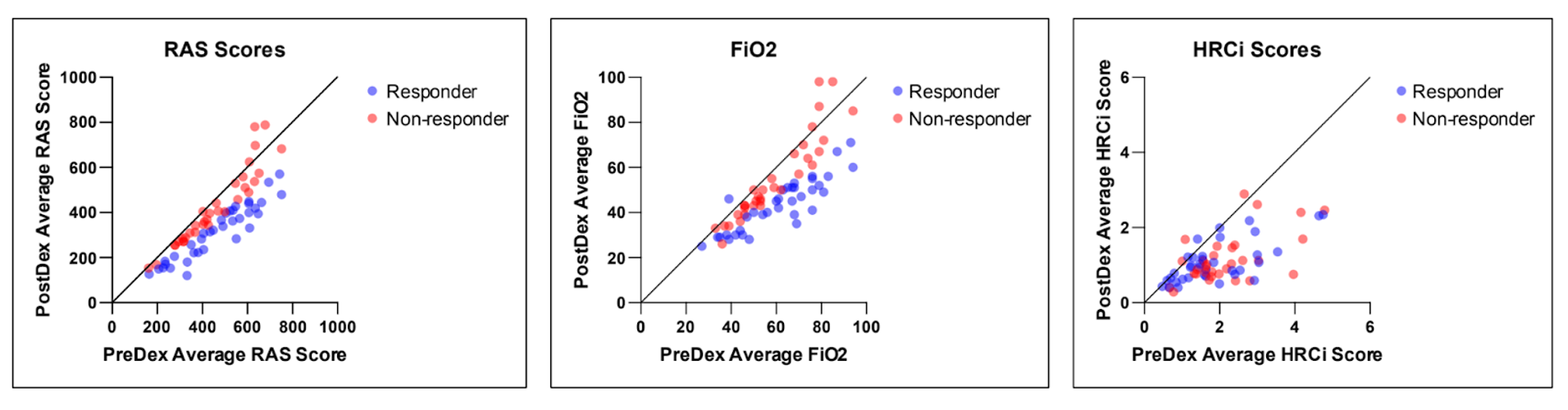
Design/Methods: Retrospective chart review of all NICU patients < 32 weeks gestational age (GA) admitted from 2012-2022 who received >3 consecutive days of dexamethasone for lung disease. Daily mean FiO2, HRCi, and RAS (respiratory support x FiO2) were calculated days -2 to +2 relative to dexamethasone start date (day 0). We defined “dexamethasone responder” as infants that demonstrated >20% decrease in mean RAS day -2 to 0 versus days +1 to +2. We compared changes in HRCi and other clinical variables in dexamethasone responders versus nonresponders (Dex-R, Dex-NR).
Results: Of 69 infants who received dexamethasone with median GA 24 weeks (range 22-31), 36 infants (52%) had a favorable RAS response and were categorized as Dex-R. Demographics, FiO2, HRCi, and RAS at dexamethasone start were similar for Dex-R and Dex-NR. Both groups had a significant decrease in RAS (-147 vs -36, p< 0.05). Dex-R had a similar median decrease in HRCi as Dex-NR (-0.50 vs -0.87, p< 0.05). HRCi change was not significantly correlated with RAS improvement (r=-0.19, p=0.11).
Conclusion(s): In this cohort of VLBW infants, dexamethasone treatment led to improvement in FiO2, HRCi, and RAS in the majority of infants, but dexamethasone response was not correlated with improvement in heart rate variability as measured by HRCi.