Hematology/Oncology
Session: Hematology/Oncology
346 - Global Mapping of Clinical Trials for Children with Cancer
Friday, May 3, 2024
5:15 PM - 7:15 PM ET
Poster Number: 346
Publication Number: 346.114
Publication Number: 346.114

Mackenzie Kelley, B.S. (she/her/hers)
Medical Student
Eastern Virginia Medical School
Norfolk, Virginia, United States
Presenting Author(s)
Background: Access to clinical trials may contribute to the disparate pediatric cancer outcomes seen globally, in which survival rates are significantly worse in low- and middle-income countries (LMICs), where most of the world’s pediatric cancer patients reside. Although there are international calls to increase access to pediatric oncology clinical trials, no work to date has comprehensively evaluated disparities in the global pediatric oncology clinical research landscape.
Objective: This work sought to describe what type of clinical research is being conducted across the world and by whom in order to characterize the current landscape of clinical trials.
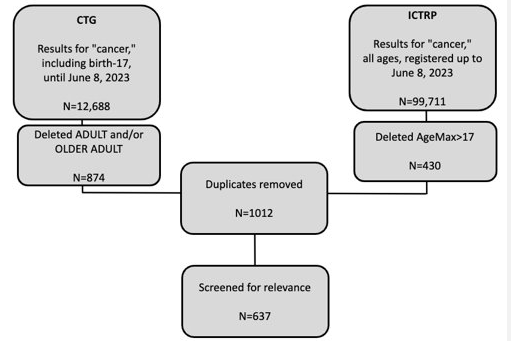
Design/Methods: Clinical studies registered in two public trial registries, ClinicalTrials.gov (CTG) and the International Clinical Trials Registry Platform (ICTRP), were screened based on the following inclusion criteria: that participants were pediatric patients (≤17 years old) diagnosed with cancer. PubMed was used to perform a literature review to identify published results. Fisher's exact test and χ2 tests were done to compare frequencies across groups.
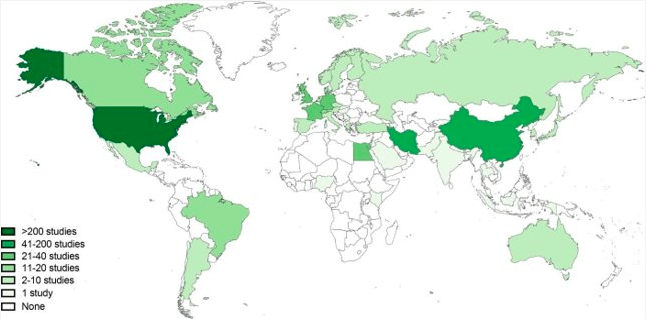
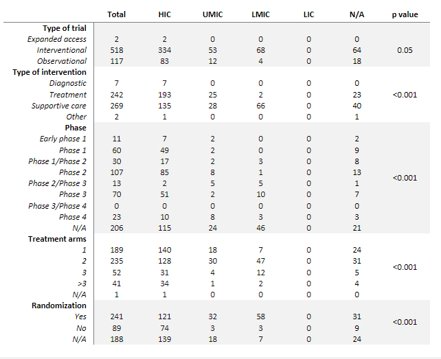
Results: The search identified 112,399 publications. A total of 637 studies met inclusion criteria. Most studies (470, 74%) were conducted in high income countries (HICs), while 167 (26%) studies were in middle-income countries, and no studies were registered in low-income countries. Study characteristics differed significantly among country income status, with HICs registering mostly early phase clinical trials focusing on cancer-directed treatment and LMICs registering later phase and supportive care-based trials (p < 0.001). Most patient accrual occurred in HICs. Further, more multi-institutional, multi-national patient accrual and collaboration occurred between HICs. Of the studies registered, 101 had a peer-reviewed publications. Studies in HICs were more likely to be published (p < 0.001). However, there was no difference found in time from study to publication between income groups (p=0.20).
Conclusion(s): Globally, LMICs are under-represented in the clinical research landscape. Clinical studies in LMICs tend to be less complex and employ less rigorous interventions. This work provides the first mapping and characterization of global pediatric oncology clinical research. Furthermore, these data describe the urgent needs to invest in clinical research infrastructure in LMICs and to foster collaborations that include LMICs leadership as a critical step in achieving equity in global pediatric cancer outcomes.