Neonatology
Session: Neonatal Neurology 8: Preclinical
319 - The Aryl Hydrocarbon Receptor is Differentially Expressed in Neutrophils of Females Acutely After Neonatal Hypoxic Ischemic Brain Injury
Monday, May 6, 2024
9:30 AM - 11:30 AM ET
Poster Number: 319
Publication Number: 319.2837
Publication Number: 319.2837

Janelle M. Korf (she/her/hers)
MD-PhD student
McGovern Medical School at the University of Texas Health Science Center at Houston
Houston, Texas, United States
Presenting Author(s)
Background: Neonatal hypoxic-ischemic brain injury (nHI) is a leading cause of pediatric disability and mortality worldwide. After nHI, males are at higher risk for adverse outcomes than females. The cause for these sex differences is unclear, but it may be due to differences in the acute inflammatory response to nHI. Emerging evidence shows that the aryl hydrocarbon receptor (AHR) is a key regulator of inflammation after stroke in adults and may be differentially expressed in females. However, the role of AHR in nHI is not well-defined.
Objective: To investigate the role of AHR in mediating sex differences in acute inflammatory response to nHI.
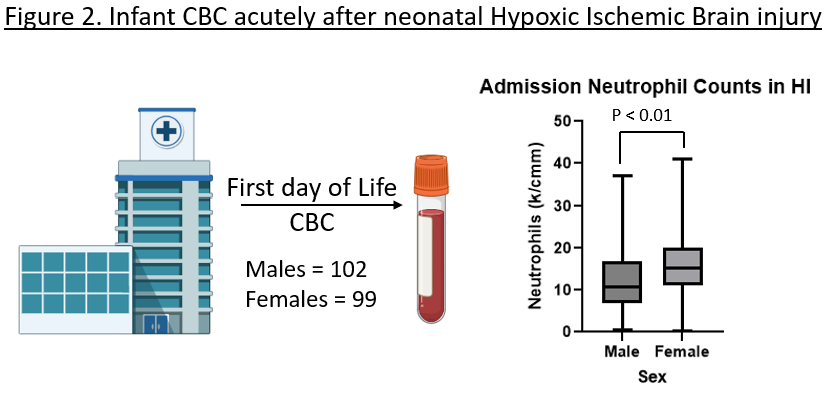
Design/Methods: We modeled nHI in pups using the modified Rice-Vannucci model (mRV), where permanent ligation of the right common carotid artery is followed by 50 minutes of hypoxia (N=6, per sex). Controls received sham surgeries followed by normoxia or hypoxia (N=4, per sex, per group). Spleens, ischemic, and contralateral brain hemispheres were collected for flow cytometry 72 hours after surgery (Fig.1A). We determined AHR levels in neutrophils, monocytes, lymphocytes, microglia, neurons, and endothelial cells. To assess immune differences in humans with nHI, we did a retrospective study at a single academic institution from 2011 to 2021 (n=310). Inclusion criteria were gestational age ≥ 36 weeks and moderate to severe nHI by Sarnat staging and CBC reported within the 1st day of life (n= 201). Statistics were performed by 2-way ANOVA with Tukey’s multiple comparisons and Mann-Whitney t-test for preclinical and clinical results, respectively; a p< 0.05 was significant.
Results: 72 hours after mRV, AHR was significantly increased in the ischemic hemisphere of females when compared to the contralateral hemisphere (p < 0.01), normoxia (p < 0.01) and hypoxia (p < 0.01) controls, and the ischemic hemisphere of males (p = 0.02). For both sexes, the majority of AHR+ cells were neutrophils (67%), with significantly more AHR+ neutrophils in females vs. male (p < 0.03). The absolute count of neutrophil in the ischemic hemisphere did not differ between sexes. In the periphery, females had a significant increase in neutrophils in the spleen compared to controls 72hrs after injury (p = 0.03); (Fig.1B-E). Similarly, in human nHI, females had a significantly higher neutrophil count than males (15.2 vs. 10.8 k/cm3, p< 0.01; Fig.2).
Conclusion(s): Sex-specific differences in the neutrophil peripheral count and AHR expression within the ischemic hemisphere indicate a sex-dimorphic role of AHR in the acute response of neutrophils to nHI.
.png)