Neonatology
Session: Neonatal Neurology 9: Preclinical
335 - Chorioamnionitis-induced Fetal Brain Injury Leads to Changes in Brain Development and Neuroimmune Regulation.
Monday, May 6, 2024
9:30 AM - 11:30 AM ET
Poster Number: 335
Publication Number: 335.3266
Publication Number: 335.3266
- AT
April W. Tan, MD (she/her/hers)
Assistant Professor of Pediatrics
University of Miami Leonard M. Miller School of Medicine
Miami, Florida, United States
Presenting Author(s)
Background: Chorioamnionitis occurs in 85% of preterm births before 30 weeks’ gestation and leads to a fetal inflammatory response syndrome (FIRS) that has adverse effects on multiple organs, including the brain. Chorioamnionitis is associated with poor neurodevelopmental outcomes in preterm infants including cerebral palsy, periventricular leukomalacia, intraventricular hemorrhage, and hearing loss. The mechanisms by which chorioamnionitis leads to brain injury are unclear.
Objective: To characterize the inflammatory profile and transcriptome response in fetal brains exposed to chorioamnionitis induced by intra-amniotic lipopolysaccharide (IA LPS) injections.
Design/Methods: Pregnant Sprague-Dawley rats were randomized into two groups to receive either 1) intra-amniotic saline injections (controls) or 2) IA LPS injections (E.coli O55:B5, 10μg per amniotic sac). IA injections were ultrasound-guided and administered on embryonic day (E) 18. C0-section was performed 24 hours after IA injections and whole brain tissue was sampled for cytokine multiplex assay and RNA sequencing.
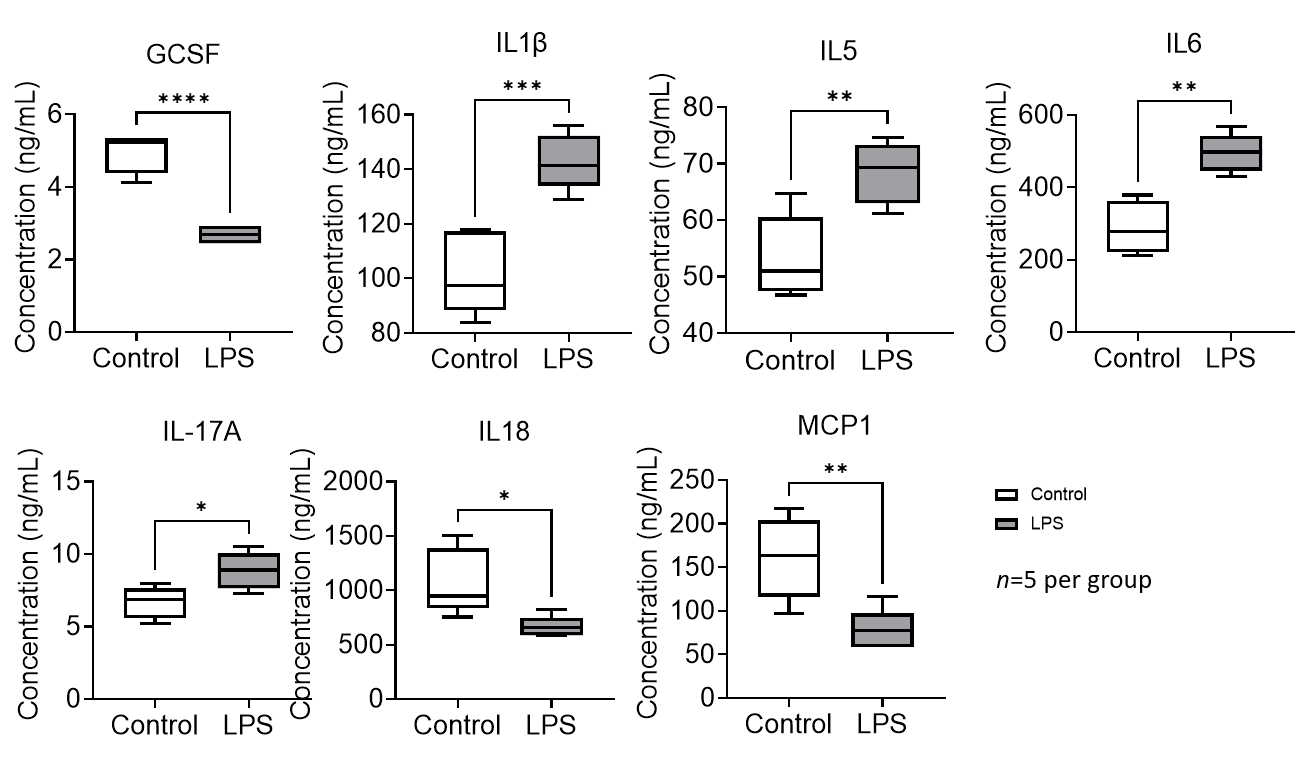
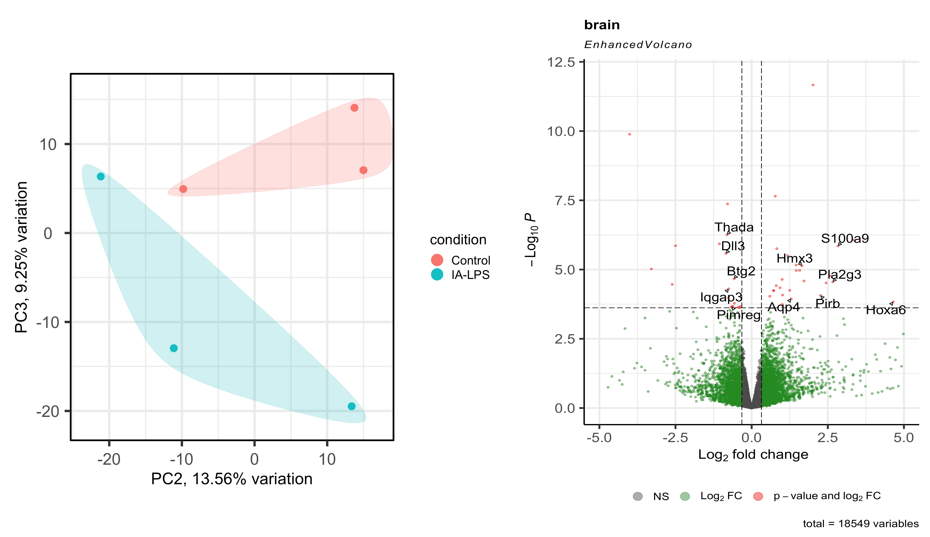
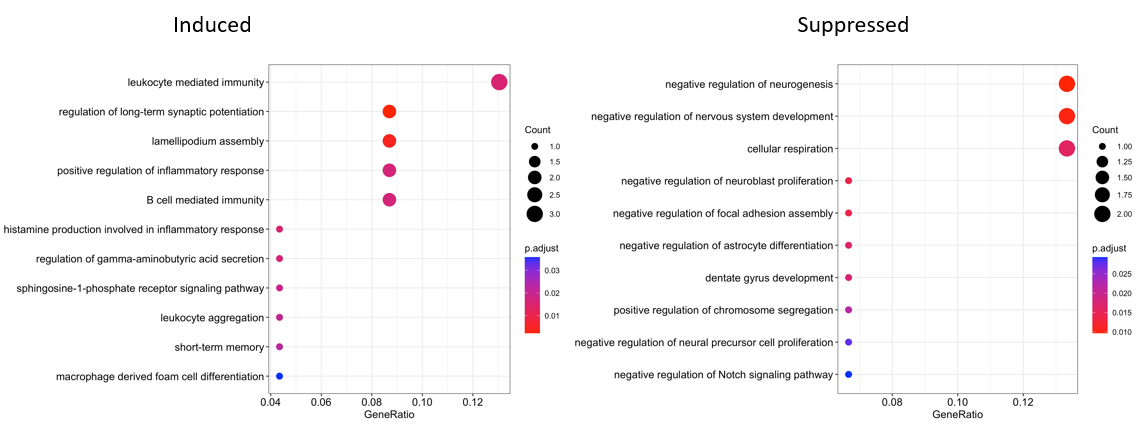
Results: LPS exposure induced brain inflammation in rat fetuses, with increased expression of pro-inflammatory cytokines IL1β, IL5, IL6 and IL17A. Brain G-CSF, IL18 and MCP1 expression were reduced in rat fetuses exposed to LPS, which are associated with neuronal growth, impaired immunoregulation and decreased blood-brain barrier integrity (Figure 1). RNA sequencing showed clear differences in gene expression between control and LPS-exposed groups. LPS induced expression of genes related to hyperactivity and abnormal behavior (Hmx3), neural development and aging (Hoxa6, Pirb), inflammation (S100a9, Apq4) and oxidative stress (Pla2g3). Genes associated with energy consumption and storage (Thada), neural development (Dll3, Btg2, Iqgap3) and immunoregulation (Pimreg) were suppressed (Figure 2). Gene ontology (GO) analysis showed differential expression of genes associated with immune activation and inflammatory cell regulation, brain excitability, memory function, neuronal growth and cell differentiation (Figure 3).
Conclusion(s): In a rat model of chorioamnionitis induced by ultrasound-guided IA LPS injections, we found fetal brain inflammation, with differential expression of genes associated with neural development and aging, memory function, immunoregulation and oxidative stress. These findings validate a small animal model of brain injury due to chorioamnionitis. Further studies will be needed to investigate specific pathways by which chorioamnionitis leads to perinatal brain injury.