Neonatology
Session: Neonatal-Perinatal Health Care Delivery: Practices and Procedures 1
442 - Minimally Invasive Surfactant Therapy (MIST) Outcomes at Northshore ISCU
Monday, May 6, 2024
9:30 AM - 11:30 AM ET
Poster Number: 442
Publication Number: 442.3240
Publication Number: 442.3240

Natasha Ahn, MD MPH (she/her/hers)
Neonatology Fellow
Comer Children's Hospital at University of Chicago Medical Center
Chicago, Illinois, United States
Presenting Author(s)
Background: Most studies on MIST have been focused on infants < 30 weeks’ gestation and although frequency of RDS does decrease with gestational age, any premature infant < 37 weeks can have RDS requiring surfactant treatment. Since its participation in the OPTIMIST trial, Northshore ISCU has continued to use MIST as its primary mechanism of delivering surfactant (poractant alpha or calfactant) to infants on CPAP, not needing emergent intubation, whose oxygen requirement >30%. Given infants were not in the trial, the gestational age (GA) was not limited and any infant with diagnosis of RDS was a candidate for MIST.
Objective: This study follows the clinical outcomes all infants that received MIST outside of OPTIMIST trial to determine if the procedure is safe and effective for infants >30 weeks GA as well as the younger population that is more well studied in the literature.
Design/Methods: Retrospective chart review of all infants who have received MIST not included in the OPTIMIST trial (6/1/2020-8/16/2023). The primary outcome was intubation within 7 days after MIST due to RDS. Secondary outcomes were BPD, IVH grade 3 or 4, PVL, NEC >= stage 2, and air leak requiring drainage.
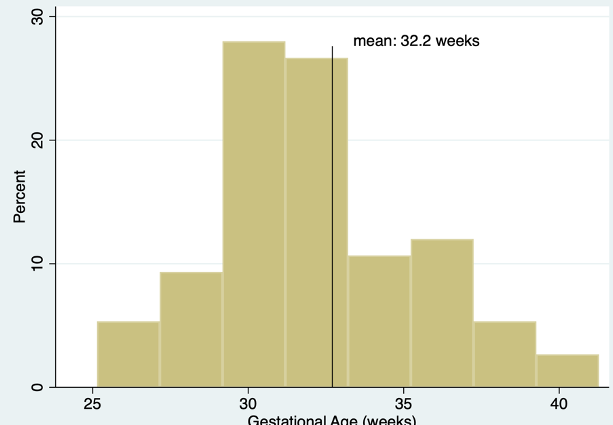
Results: 73 patients were administered surfactant (poractant alpha or calfactant) via MIST. The GA range of infants receiving MIST was 25.1-41.3 weeks and mean was 32.2 weeks (Figure 1). Mean BW was 1886.4 grams, ranging from 460g to 4340g. 7 infants (9.6%) who received MIST were intubated within 7 days for RDS (Table 1). There was no significant difference in GA, BW, PEEP or FiO2 at time of MIST between those who were intubated at 7 days and those who were not. There was no difference in intubation rates based on surfactant type. When comparing infants of GA <=30 week and >30 weeks, there was no significant difference between rates of intubation at 7 days or air leak, or BPD (although only infants GA < 32 were eligible for diagnosis). There was as expected higher rates PVL and NEC in the < 30wk GA (Table 2) with rates comparable to historical VON data from this institution.
Conclusion(s): Surfactant delivery via MIST for infants >30 weeks GA is feasible. This case series suggests MIST does not cause acute decompensation and sheds light on the outcomes of MIST used in older infants with RDS.
.png)
.png)
