Immunizations/Delivery
Session: Immunizations/Delivery 2
51 - Human in vitro modeling characterizes mechanism of action of adjuvantation systems defining scalable and affordable precision vaccine formulations for early childhood
Saturday, May 4, 2024
3:30 PM - 6:00 PM ET
Poster Number: 51
Publication Number: 51.1419
Publication Number: 51.1419
- ST
Sanya Thomas, MD, MPH, MMSc
Research Fellow
Boston Children's Hospital
Boston, Massachusetts, United States
Presenting Author(s)
Background: Children demonstrate distinct immunity in early life including diminished Th1-polarizing cytokine production contributing to high susceptibility to infections caused by intracellular pathogens such as respiratory viruses.
Objective: This challenge also pertains to pediatric vaccine discovery and development, and could be overcome by developing precision adjuvantation systems with well characterized mechanisms of action tailored to enhance age-specific immunogenicity.
Design/Methods: To this end, we employed novel age-specific human in vitro assays to characterize cellular and molecular activities of a selection of adjuvants in children aged between 2-4 years in soluble, oil-in-water and liposomal formulations, including several developed for global open access.
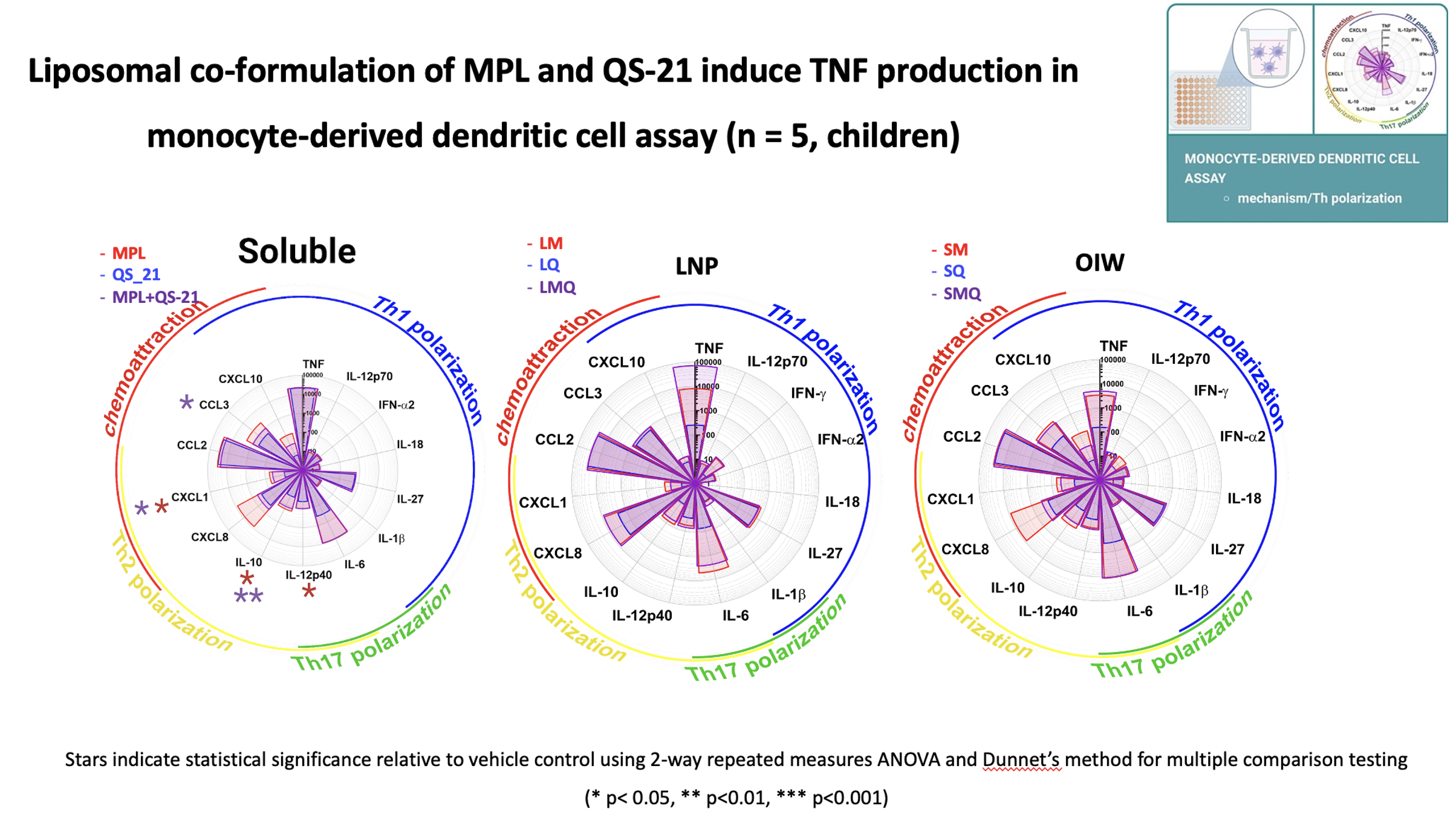
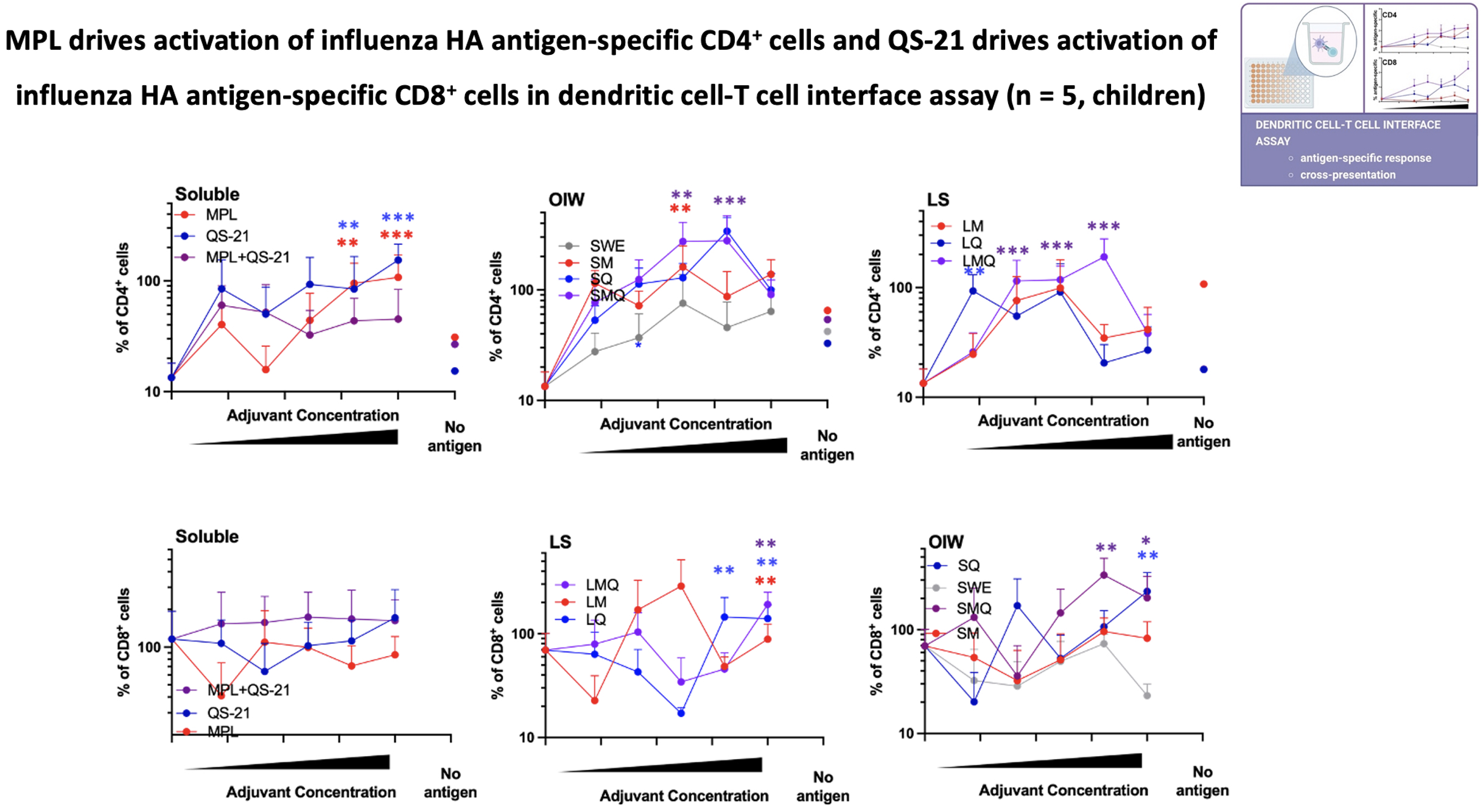
Results: In a whole blood assay, which predicts the reactogenicity potential of adjuvants, formulations containing the TLR4 agonist, monophosphoryl lipid A (MPL), potently induced an innate immune response with primary activation of monocytes, and liposomal formulations were more selective in inducing cytokine production compared to soluble and oil-in-water formulations in children as well as adults. In a monocyte-derived dendritic cell (MoDC) assay, which dissects the mechanism by which adjuvants activate differentiation of T helper (Th) cell subsets, liposomal formulations activated MoDCs to produce Th1-polarizing cytokine response, which is important for anti-viral host defense, with more robust TNF induction observed in children than adults. In a DC-T cell interface assay that demonstrates antigen processing and presentation, activation of antigen-specific CD4+ T cells was driven by MPL-containing formulations and that of CD8+ T cells was induced by the adjuvant QS-21 with greater variability observed in children than in adults.
Conclusion(s): Insight into the mechanisms of action of adjuvanted vaccine formulations via age-specific human in vitro modeling may advance global health by accelerating and de-risking development of affordable and scalable precision-adjuvanted vaccines.
.jpg)