Hematology/Oncology
Session: Hematology/Oncology
351 - Minority Participation in Clinical Trials: Lessons from Sickle Cell Disease
Friday, May 3, 2024
5:15 PM - 7:15 PM ET
Poster Number: 351
Publication Number: 351.532
Publication Number: 351.532

Sindy K. Mora Garavito, MD (she/her/hers)
Research Coordinator
NYC H+H/Jacobi Medical Center
Yonkers, New York, United States
Presenting Author(s)
Background: The underrepresentation of minorities in clinical trials limits their generalizability to diverse populations and impedes the development of equitable healthcare solutions. We often attribute these disparities to individual-level factors such as mistrust, fear of being experimented on, socioeconomic status, and cultural barriers. However, insights from clinical trial enrollment for sickle cell disease, most prevalent in minorities, show that we cannot explain underrepresentation by their "unwillingness to participate." Disparity research highlights the importance of "place" in healthcare inequities, suggesting that access to clinical trials at minority-serving healthcare institutions is a crucial systems factor.
Objective: To evaluate minority participation in pharmaceutical-sponsored sickle cell clinical trials at an inner-city public hospital.
Design/Methods: We retrospectively analyzed enrollment data from sickle cell disease clinical trials at the public hospital between 2021 and 2023.
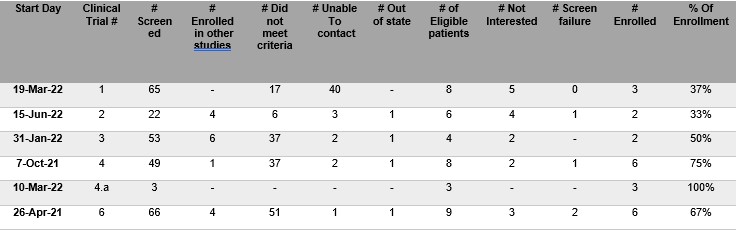
Results: During this period, we initiated six pharmaceutical-sponsored clinical trials, enrolling 22 patients aged between 04 years and 65 years. We operationally defined "willingness to participate" as the ratio between subjects enrolled and the number eligible. Participation ranged from 33% to 100%. A comprehensive summary of the enrollment data is presented in the accompanying table.
Conclusion(s): Our findings suggest that providing access to clinical trials in community hospitals is critical to increasing minority enrollment. Despite the proximity of major academic medical centers, many minority patients choose to seek care at public hospitals, which highlights the importance of offering clinical trials at these hospitals. We contend that prioritizing place is pivotal for reducing systemic healthcare disparities and ensuring the generalizability of clinical research.