Neonatology
Session: Neonatal Fetal Nutrition & Metabolism 3: Enteral Nutrition
441 - Iron Concentration Affects Iron Absorption in Fetal Intestinal Epithelial Cells
Sunday, May 5, 2024
3:30 PM - 6:00 PM ET
Poster Number: 441
Publication Number: 441.2014
Publication Number: 441.2014

Rico Carter, II, MS (he/him/his)
Medical Student (MS1)
Wright State University Boonshoft School of Medicine
Tampa, Florida, United States
Presenting Author(s)
Background: Preterm infants require iron supplementation after birth to prevent iron deficiency. Clinical observations show that the incidence of low iron storage remains high even when infants are given high oral iron dose. In mature intestinal epithelial cells, transferrin receptor (TfR) and Divalent metal transporter 1 (DMT1) contain 3’ iron responsive elements (IRE+) in their mRNA transcript. In iron cellular deficient stage, these transcripts are stabilized by Iron Regulatory Proteins (IRPs) binding to the IREs which protects the mRNA against nucleolytic degradation to make DMT-1 and TfR to bring more iron into the cells. It is unknow if this process is similar in immature intestinal epithelial cells.
Objective: We aim to study the effects of iron availability on cellular iron absorption regulation using in-vitro cell culture of fetal intestinal epithelial cells.
Design/Methods: Fetal intestinal epithelial cells (FH74-int, ATCC CCL-241) were exposed to FeCl3 at 0 to 200 μM in the growth media in an increment of 50 μM. Relative gene expression of 3’ TfR and DMT1-IRE+ genes were calculated using RTq-PCR. Intracellular iron content was also measured using inductively coupled plasma atomic emission spectroscopy (ICP-AES) and normalized by total cellular protein using Bradford assay. Three separate experiments were performed in triplicates and the three biological replicates gene expression means were compared using student t-test with p< 0.05 considered significant.
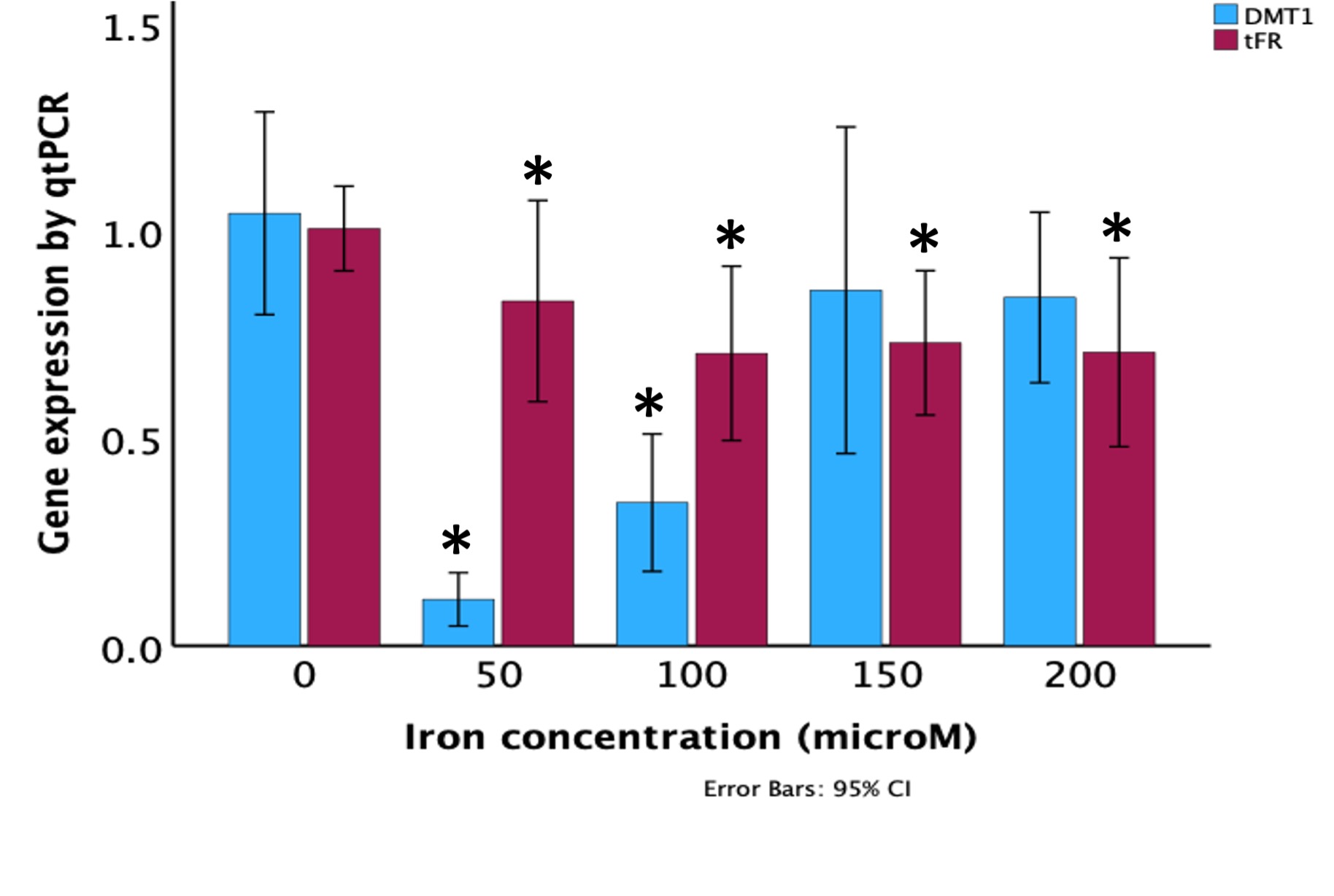
Results: Intracellular iron content was significantly higher than negative control at 50, 100, and 150 μM with the highest intracellular iron content at 50 and 100 μM (Figure 1). TfR was down regulated at all iron concentrations. DMT1-IRE+ was down regulated at 50 and 100 μM but not significantly at 150 and 200 μM iron concentrations (Figure 2).
Conclusion(s): Iron availability to certain concentration improved intracellular iron uptake and intracellular iron sufficiency down regulated DMT1-IRE+ and TfR in fetal intestinal epithelial cells. This implies intestinal iron absorption is optimal at a range of iron availability and above that range, iron absorption may not improve. Premature intestinal cells exhibited similar iron dependent TfR and DMT1-IRE+ regulations as in mature cells. These results provide insight into how best to prevent iron deficiency in preterm infants.
.jpg)