Neonatology
Session: Neonatal Neurology 2: Clinical
24 - Magnetic resonance assessment of brain Injury after early and extended erythropoietin monotherapy in hypoxic ischemic encephalopathy: a multicentre double-blind pilot randomised controlled trial
Friday, May 3, 2024
5:15 PM - 7:15 PM ET
Poster Number: 24
Publication Number: 24.93
Publication Number: 24.93

Reema Anil Garegrat, DM MRCPCH (she/her/hers)
Clinical Research Fellow in Neonatal Neurology
Imperial College,London
london, England, United Kingdom
Presenting Author(s)
Background: Although erythropoietin treatment 24 h after birth is not neuroprotective for moderate or severe hypoxic ischemic encephalopathy (HIE), clinical effects of early administration are not known.
Objective: To examine the feasibility of early and extended erythropoietin monotherapy after HIE using magnetic resonance imaging (MRI).
Design/Methods: We conducted a double-blind pilot randomised controlled trial (RCT) recruiting neonates born at or after 36 weeks with moderate or severe neonatal HIE from seven tertiary neonatal units in South Asia between 31 Dec 2022 and 3 May 2023. Neonates were randomly assigned to erythropoietin (500U/kg) or to the placebo (sham injections using a screen) within 6 hours of birth and then daily for 8 days using a centralised web-based program stratified for severity of encephalopathy and centre. The clinical and research teams involved in the data analysis or outcome assessments (including central readers of MRI) except the designated study research nurse administering the study drug, were masked to the allocation. MRI was performed at 2 weeks of age. The primary outcome was the feasibility of early erythropoietin administration.
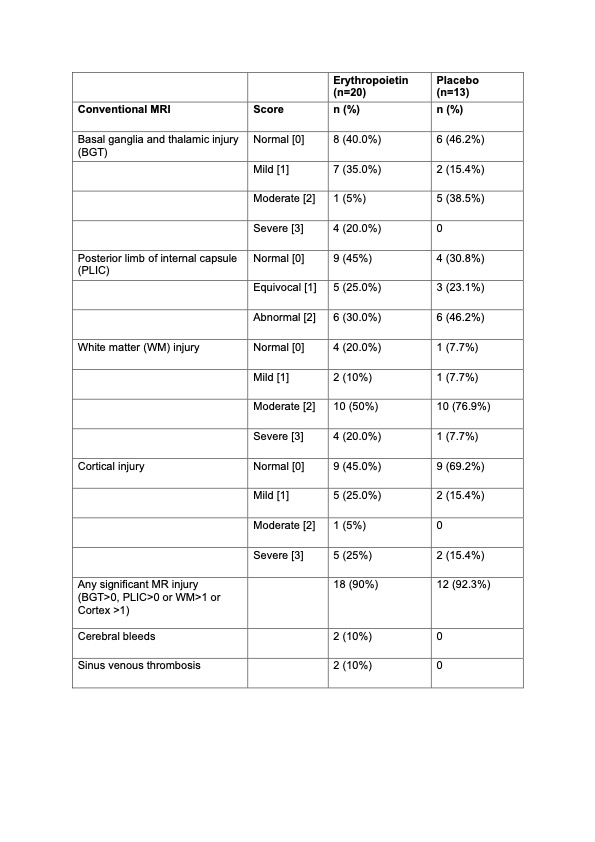
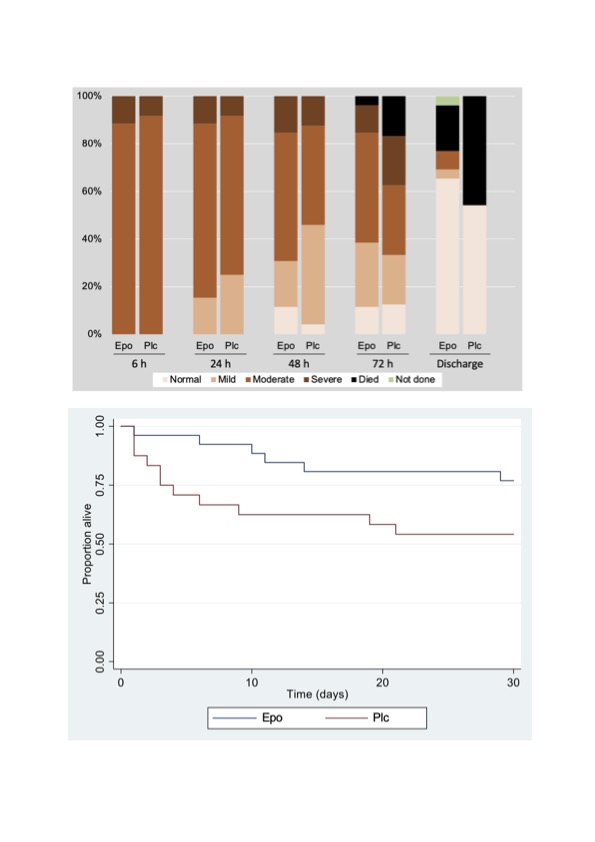
Results: Of the 56 eligible babies, 50 were recruited (45 had moderate and 5 had severe HIE at randomisation (Figure 1). Mean age at first dose was 4.2 (SD 1.1) hours. Five (19%) of 26 infants allocated to erythropoietin and 11 (46%) of 24 allocated to placebo died before hospital discharge (Table 1). Among 34 babies who survived to discharge, MRI was performed in 33 (97.1%; 20 in erythropoietin and 13 placebo). In the erythropoietin and placebo groups, (Table 2) moderate or severe injury to basal ganglia, white matter and cortex occurred in 5 (25%) vs 5 (38.5%); 14 (70%) vs 11 (85%); and 6 (30%) vs 2 (15.4%) respectively. Signal intensity in the posterior limb of internal capsule was equivocal or abnormal in 11 (55%) neonates in the erythropoietin and 9 (69.2%) in the placebo group. Sinus venous thrombosis was seen in 2 (10%) neonates in the erythropoietin group and none in the placebo group. Development of both infants and repeat MRI (performed in 1 infant) at 6 months were normal. Survival at 6 months occurred in 19 (73%) of erythropoietin and 13 (54%) placebo group.
Conclusion(s): Administration of early and extended erythropoietin therapy is feasible; however, 2 infants in the erythropoietin group had thrombosis that resolved. A clinical trial evaluating safety and efficacy of erythropoietin neuroprotection in south Asia is planned.
Clinical Trials.gov ID: NCT05395195
.jpg)