Neonatology
Session: Neonatal Neurology 2: Clinical
25 - Title: Characteristics of Infants Treated with Therapeutic Hypothermia in US NICUs 2008-2022
Friday, May 3, 2024
5:15 PM - 7:15 PM ET
Poster Number: 25
Publication Number: 25.91
Publication Number: 25.91

Kaashif A. Ahmad, MD (he/him/his)
National Research Director of Neonatal Clinical Trials
Pediatrix Medical Group
Houston, Texas, United States
Presenting Author(s)
Background: Therapeutic hypothermia (TH) has become the standard treatment strategy in high income countries for infants >= 36 weeks’ gestation with hypoxic-ischemic encephalopathy (HIE). However, there is little data about the implementation of widespread TH and possible changes in the clinical characteristics of infants who receive TH.
Objective: Our objective is to describe the population of infants receiving TH in a large group of US neonatal intensive care units (NICUs) with neonatal encephalopathy (NE).
Design/Methods: This is a retrospective cohort study of patients from the Pediatrix Clinical Data Warehouse (CDW), a HIPAA compliant, deidentified dataset, between 2008 and 2022. Data in the CDW are entered prospectively by clinicians and deidentified before aggregation. We describe and compare infant demographics and diagnoses over time with descriptive statistics.
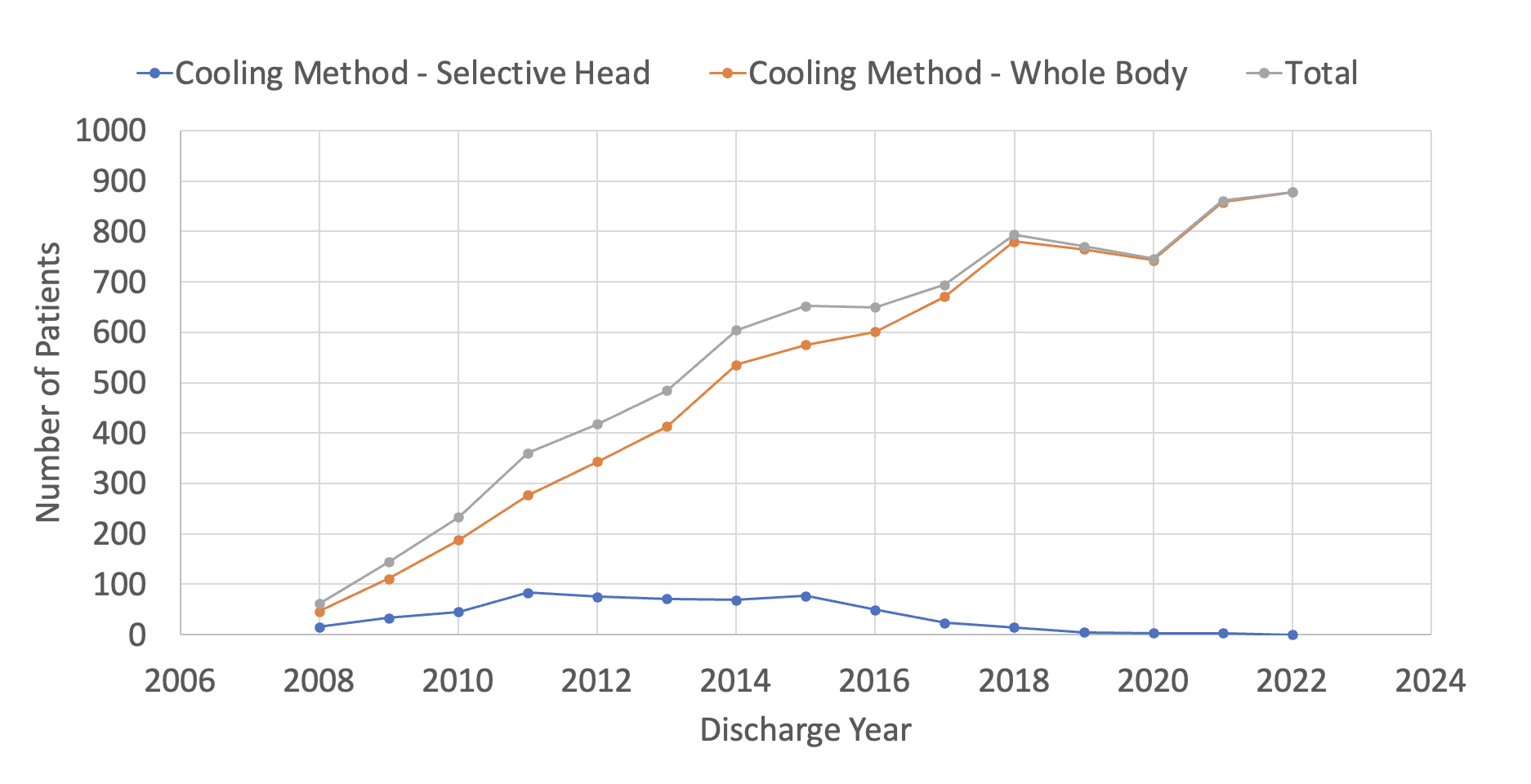
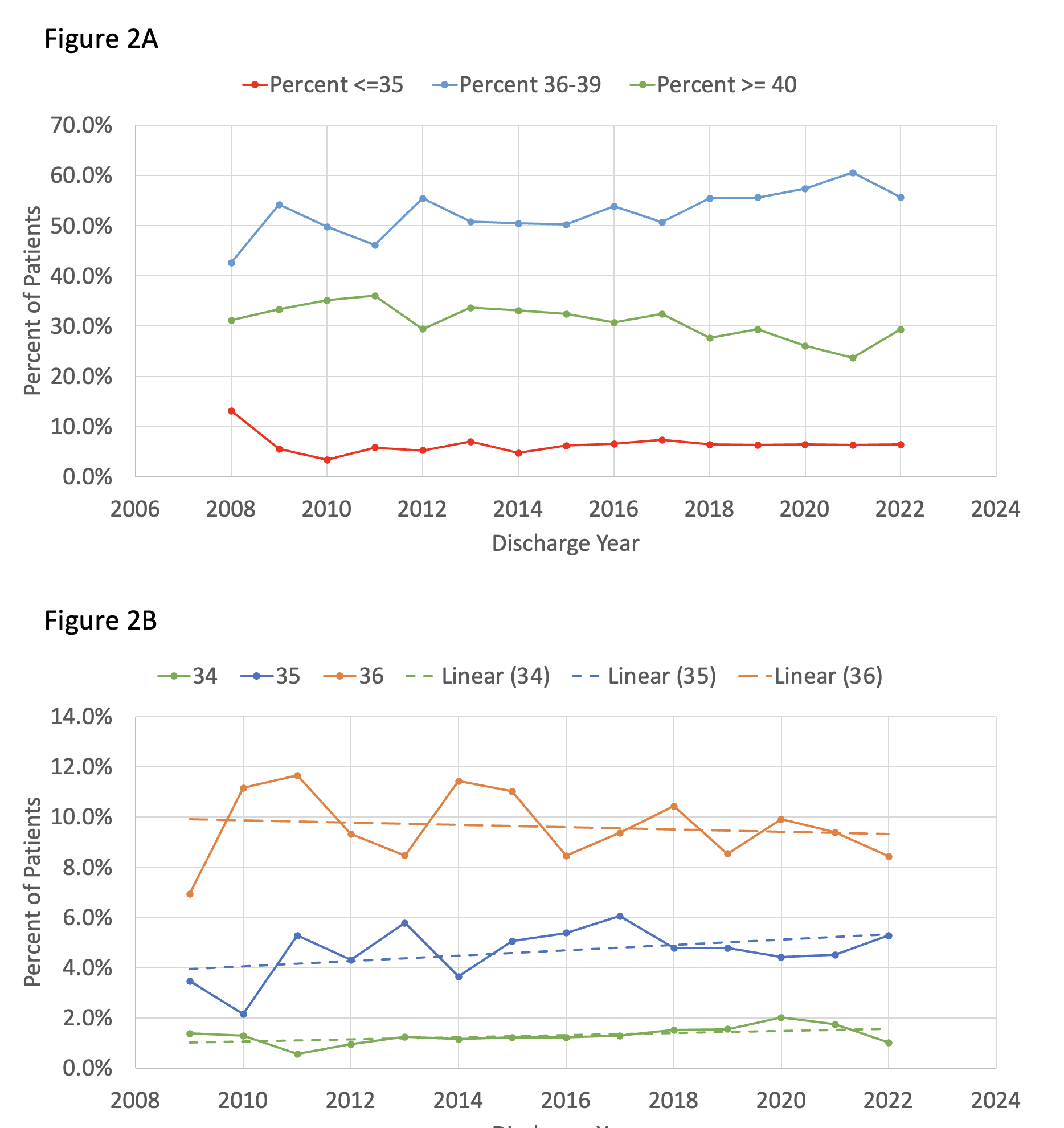
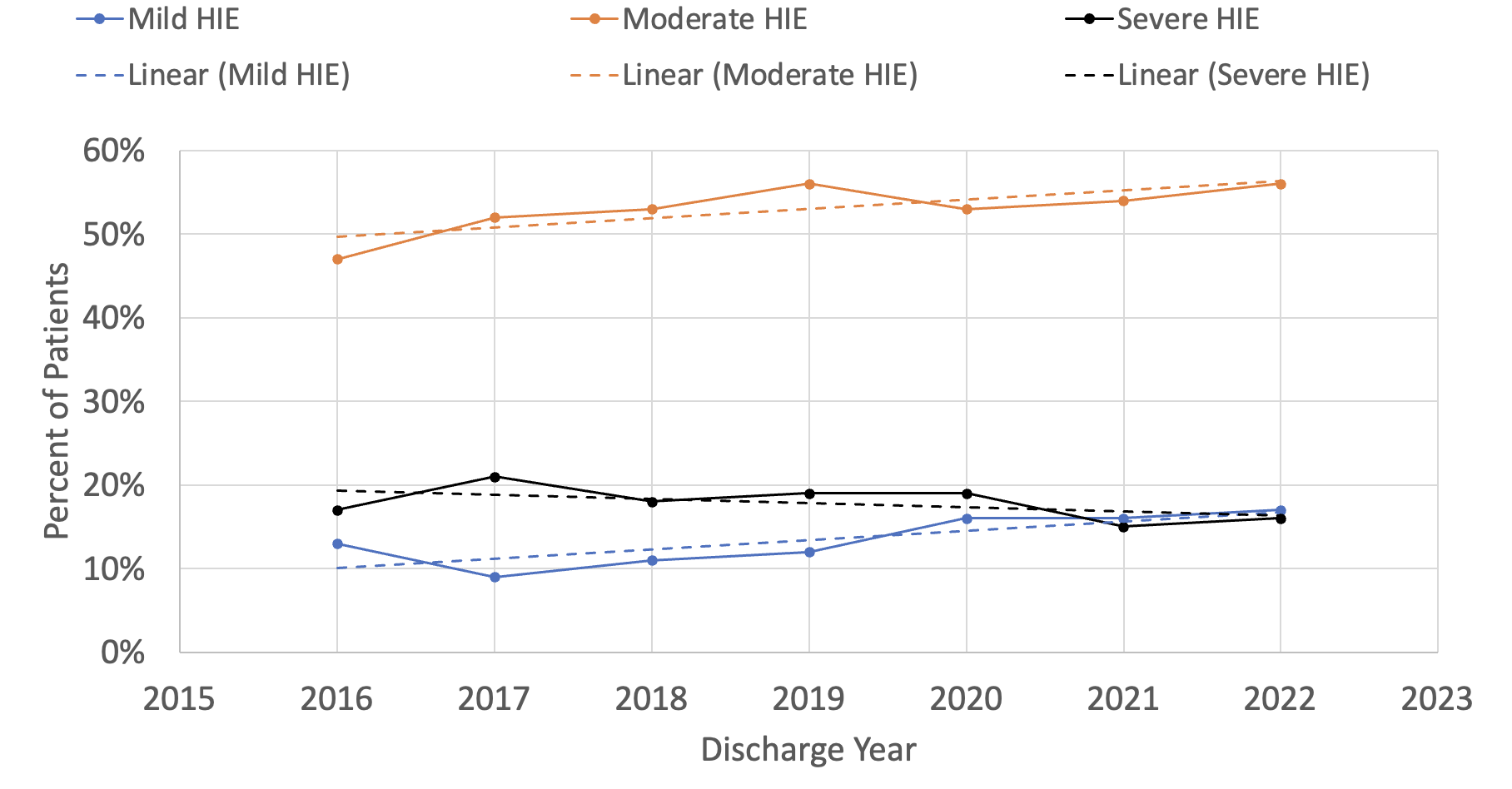
Results: Of 1,320,831 patients entered into the CDW from 2008 – 2022, we identified 8,349 who received TH during their NICU stay. Within the CDW, the use of TH has increased substantially over time, from 61 patients in 2008 to 878 in 2022 (Figure 1A), representing a 14-fold increase in TH utilization. Total body cooling accounted for the overwhelming majority of TH 7,784 (93%) and after 2018, less than 10 patients per year received selective head cooling. Since 2009, less than 10% of all patients receiving TH were below 36 weeks’ gestational age (Figure 1B), with a modest increase in the percentage of these patients over the past 10-years (Figure 1C). ICD-10 allowed greater detail with regards to HIE diagnosis starting in 2018. Since that time, moderate hypoxic-ischemic encephalopathy (HIE) has consistently been the most common diagnosis associated with TH, with between 47-56% per year (Figure 2). Of all patients receiving TH, those with a diagnosis of mild HIE has been steadily increasing and reached 17% in 2022. We plan to further evaluate the baseline demographics, clinical characteristics and medication use in this population.
Conclusion(s): Over the past 15 years, TH use has increased significantly, with a shift to almost exclusive whole-body cooling. Most infants who receive TH are >= 36 weeks’ gestational age with a diagnosis of moderate encephalopathy. However, some infants with a gestational age < 36 weeks or a diagnosis of mild encephalopathy also receive TH, suggesting an expansion of TH outside of the population described in the pivotal trials.