Neonatology
Session: Neonatal Neurology 8: Preclinical
325 - Intrauterine growth restriction alters oligodendrocyte metabolism
Monday, May 6, 2024
9:30 AM - 11:30 AM ET
Poster Number: 325
Publication Number: 325.3039
Publication Number: 325.3039

Jill Chang, MD (she/her/hers)
Assistant Professor
Children's Hospital Colorado
Aurora, Colorado, United States
Presenting Author(s)
Background: Intrauterine growth restriction (IUGR) is defined as a significant reduction in fetal growth resulting in birth weight < 10th percentile for gestational age. IUGR leads to increased risk of mortality and morbidities including cerebral palsy (CP). White matter injury (WMI) (the histopathologic correlate of CP) has been demonstrated in IUGR. Previous animal studies have shown impaired oligodendroglial (OL) differentiation as a likely cause of WMI in IUGR, however mechanisms of blocked maturation remain incompletely understood.
Objective: To identify OL specific transcriptional changes due to IUGR that result in WMI.
Design/Methods: Ribotag method was used to isolate OL specific RNA. Ribotag mice contain an epitope-tagged ribosomal protein allele that is activated in specific cell types by mating to a Cre-recombinase mouse. Conditional activation of this allele leads to epitope-tagged polyribosomes that can be purified from the target cell population via immunoprecipitation. PDGFRaCreER mice were used as PDGFRa is expressed in oligodendrocyte progenitor cells (OPCs), which are known to be most susceptible to perinatal brain injury. To model IUGR, micro-osmotic pumps containing a potent vasoconstrictor, thromboxane A2 analog, were implanted in pregnant dams at E12.5. After spontaneous birth, pups weighing < 10th percentile were categorized as IUGR. Tamoxifen was injected in pups subcutaneously at P0 to activate Cre-recombination. Tissue collection and total RNA isolation was performed at P14. RNA libraries were constructed and sequenced. Differential expression was determined using DESeq2, FDR-adjusted p-value < 0.05 cutoff for determining significance. Data was analyzed using BioJupies and Ingenuity pathway analysis programs. Validation was performed with RT qPCR. Unpaired t-tests were used to analyze all data, p< 0.05 level of significance.
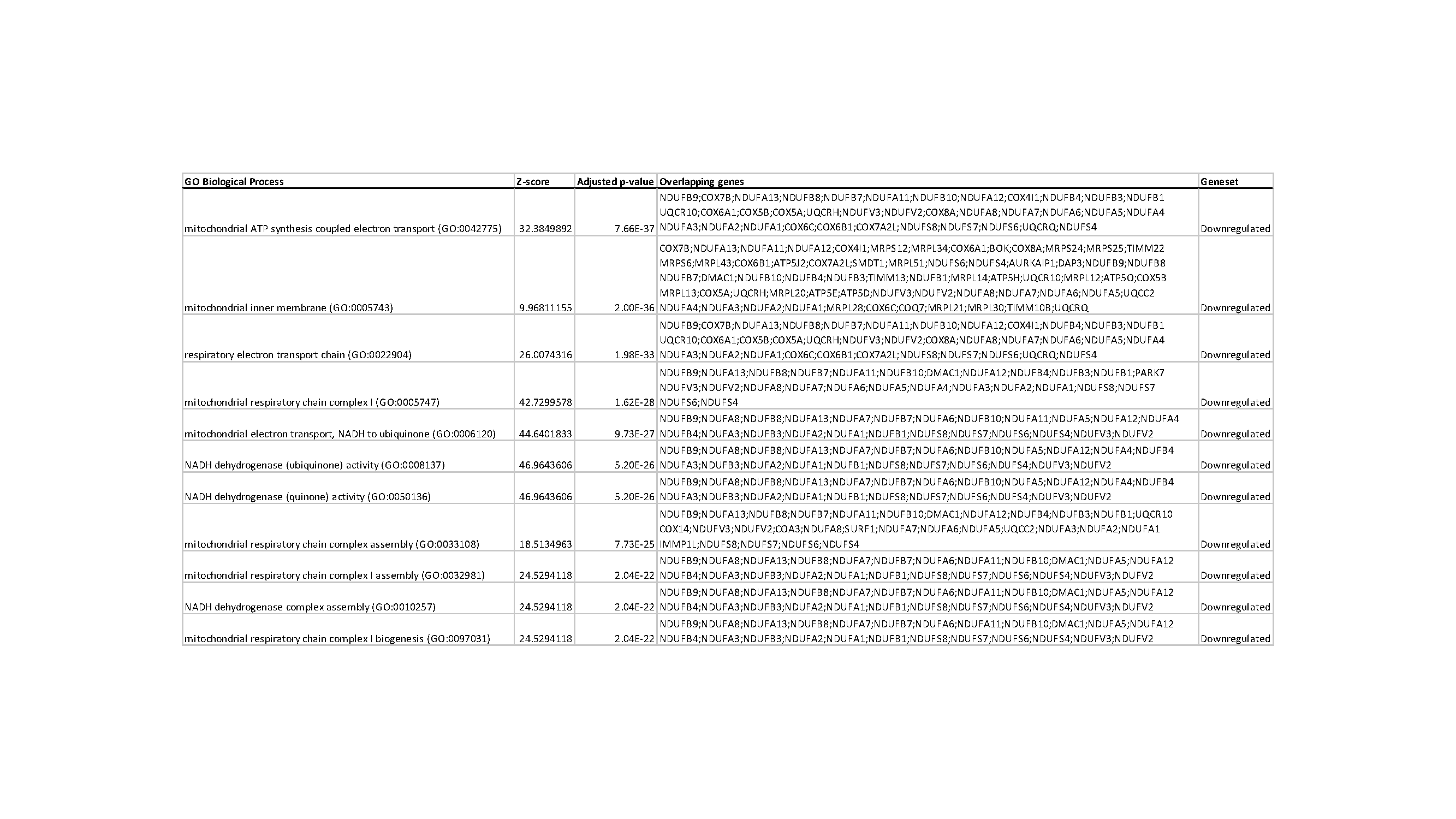
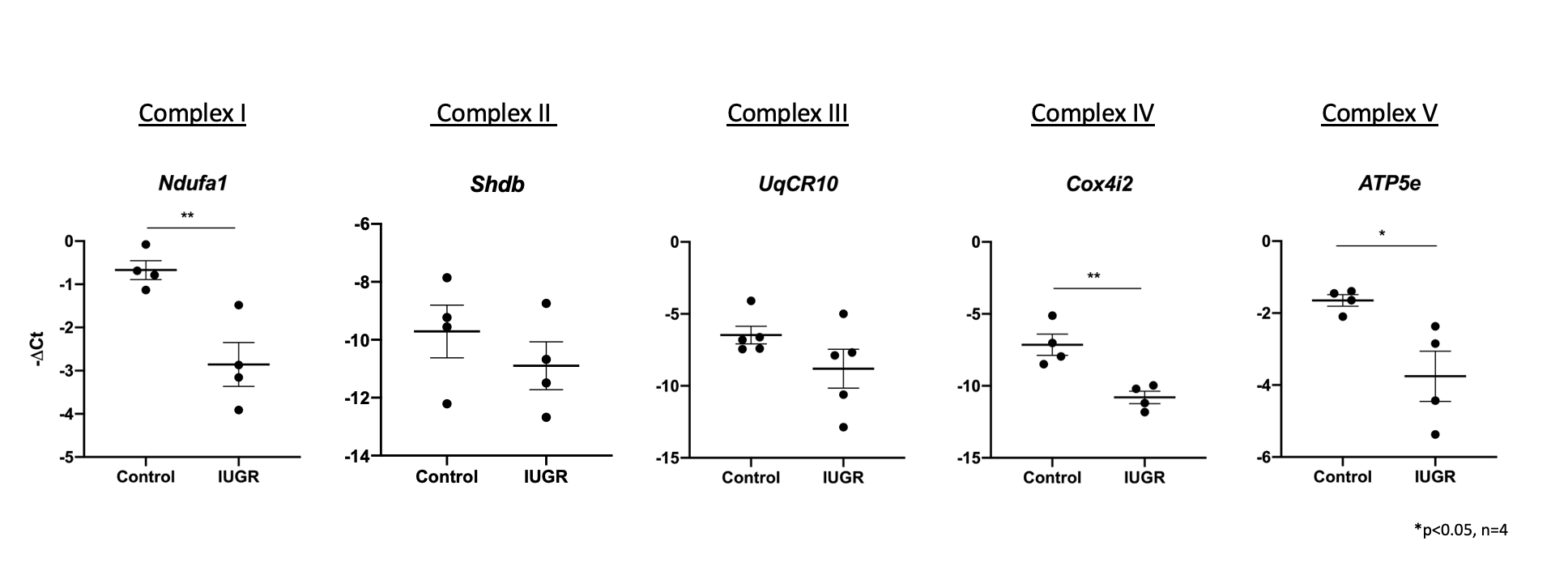
Results: There was a significant downregulation of genes involved in mitochondrial ATP synthesis, specifically the electron transport chain (ETC) in IUGR compared to control (p < 0.05, n=4) (Figure 1 and Table 1). Validation with qRT-PCR was performed with genes in ETC (p < 0.05, n=4) (Figure 2).
Conclusion(s): These findings demonstrate evidence of OL metabolic adaptation following IUGR. Specifically, a decrease in oxidative phosphorylation that may result in impaired OL maturation and decreased myelination. These results provide a potential mechanism for the WMI in IUGR. Similar findings have been documented in skeletal muscle and liver in IUGR, as well as in demyelinating diseases such as multiple sclerosis.
Figure 1.jpeg