Neonatology
Session: Neonatal Pulmonology - Clinical Science 5: New-Old ideas, Stem Cells
573 - Umbilical Cord Blood-Derived Cell Therapy for Preterm Lung Injury: A Systematic Review and Meta-Analysis of Preclinical and Clinical Studies
Monday, May 6, 2024
9:30 AM - 11:30 AM ET
Poster Number: 573
Publication Number: 573.3205
Publication Number: 573.3205

Lindsay Zhou, MBBS (he/him/his)
Neonatologist
Monash University
Clayton, Victoria, Australia
Presenting Author(s)
Background: Lung injuries, such as bronchopulmonary dysplasia (BPD), remain a major complication of preterm birth, with limited therapeutic options. One potential emerging treatment is umbilical cord blood-derived cell (UCBC) therapy.
Objective: We aimed to systematically assess the safety and efficacy of UCBC therapy for preterm lung injury in preclinical and clinical studies.
Design/Methods: A systematic search of MEDLINE, Embase, CENTRAL, ClinicalTrials.gov and WHO International Trials Registry Platform was performed. A meta-analysis was conducted with Review Manager (5.4.1) using a random effects model. Data was expressed as standardised mean difference (SMD) for preclinical data and relative risk (RR) for clinical data, with 95% confidence intervals (CI). Potential effect modifiers were investigated via subgroup analysis. Certainty of evidence was assessed using the GRADE approach.
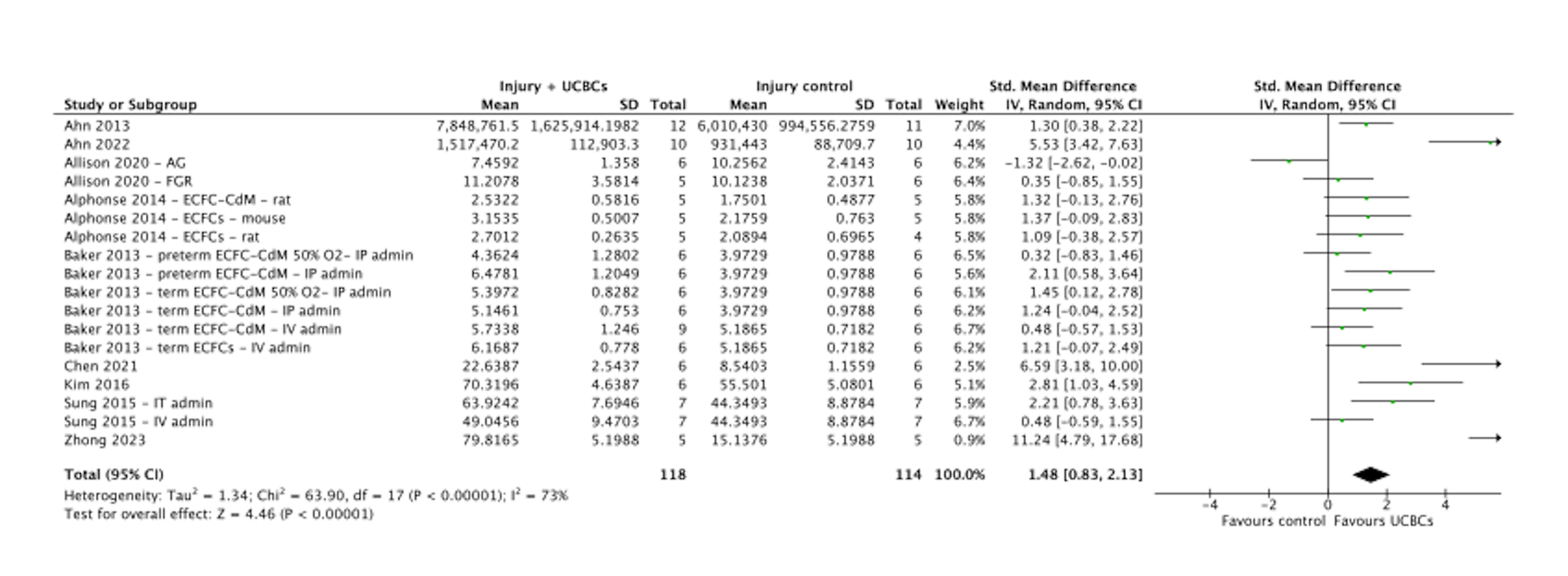
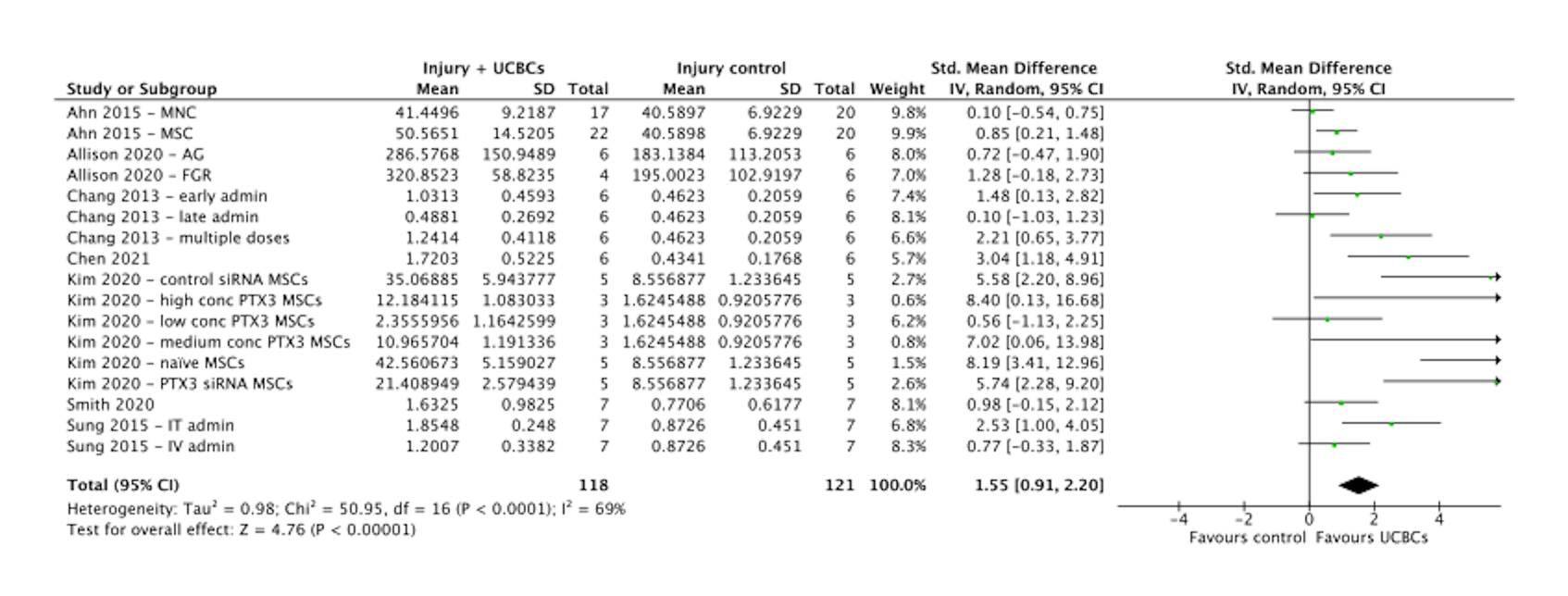
Results: 1625 citations were identified and after screening, 21 preclinical studies and 4 clinical studies met eligibility criteria. Statistically significant improvements were seen across several preclinical outcomes, including alveolarization (SMD, 1.31, 95%CI 0.97 to 1.64, P < 0.00001, Figure 1), angiogenesis (SMD, 1.48, 95%CI 0.83 to 2.13, P < 0.00001, Figure 2) and anti-inflammatory cytokines (SMD, 1.55, 95%CI 0.91 to 2.20, P < 0.00001, Figure 3). No significant difference was seen in the development of BPD in the clinical studies following UCBC administration (RR, 0.88, 95%CI [0.56, 1.40] P = 0.59). Across both preclinical and clinical studies, administration of UCBCs appeared safe. Certainty of evidence was assessed as ‘low’.
Conclusion(s): Administration of UCBCs was associated with significant effects across several lung injury markers in preclinical studies. Early clinical studies demonstrated UCBC therapy as safe and feasible but lacked extensive data regarding efficacy.
.png)