Neonatology
Session: Neonatal Neurology 1: Clinical
14 - Temporal profile and association of RNA-binding motif protein 3 (RBM3) with hypoxic-ischemic encephalopathy and adverse outcomes.
Friday, May 3, 2024
5:15 PM - 7:15 PM ET
Poster Number: 14
Publication Number: 14.155
Publication Number: 14.155

Constance Burgod, BSc, MRes (she/her/hers)
PhD Student
Imperial College London
London, England, United Kingdom
Presenting Author(s)
Background: RNA-binding motif protein 3 (RBM3) is a molecular marker of hypothermia with potential neuroprotective effects in stroke and other neurodegenerative diseases but has not been explored in hypoxic-ischemic encephalopathy (HIE).
Objective: To examine the temporal course and relations of RBM3 with HIE and adverse outcomes.
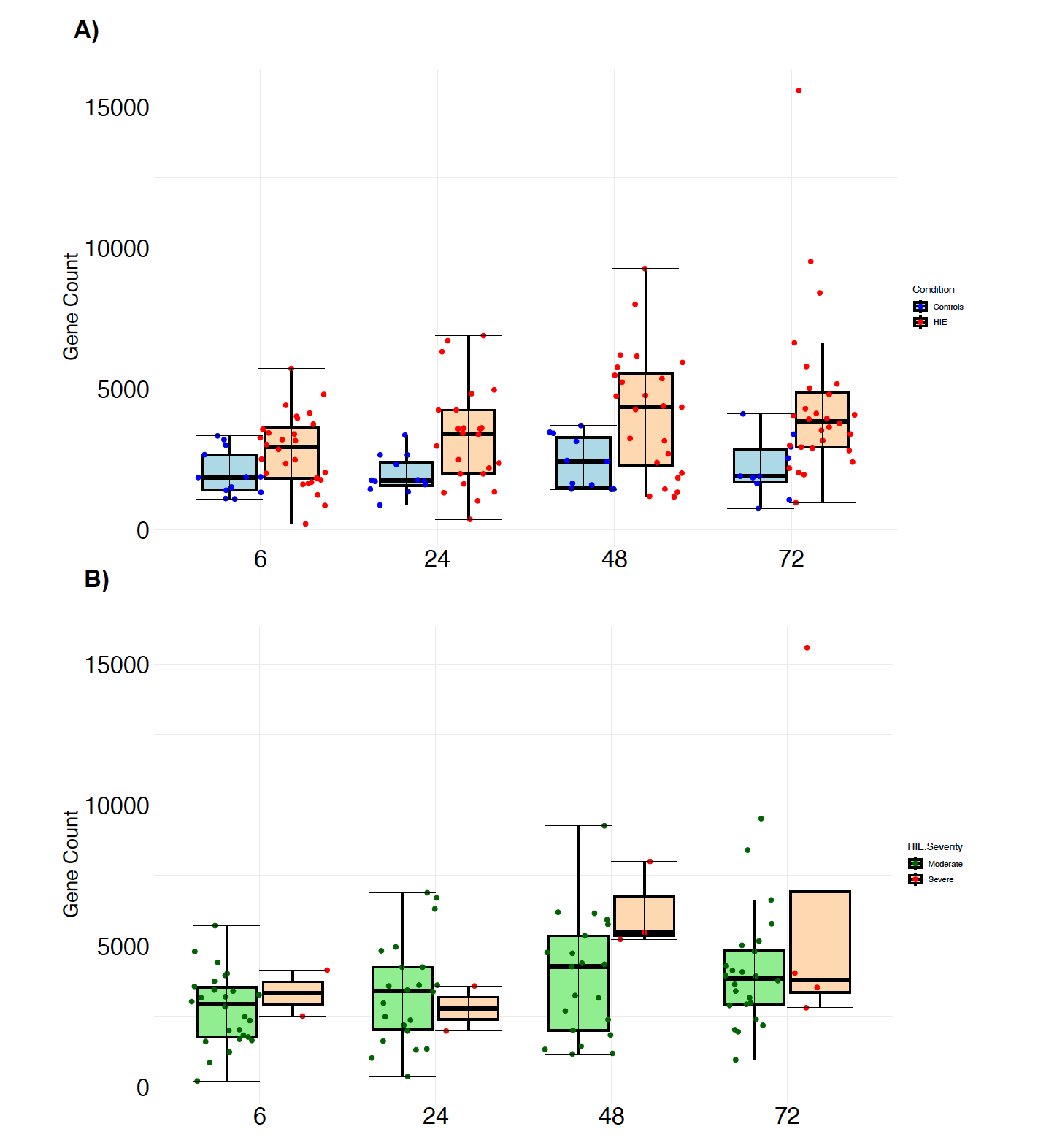
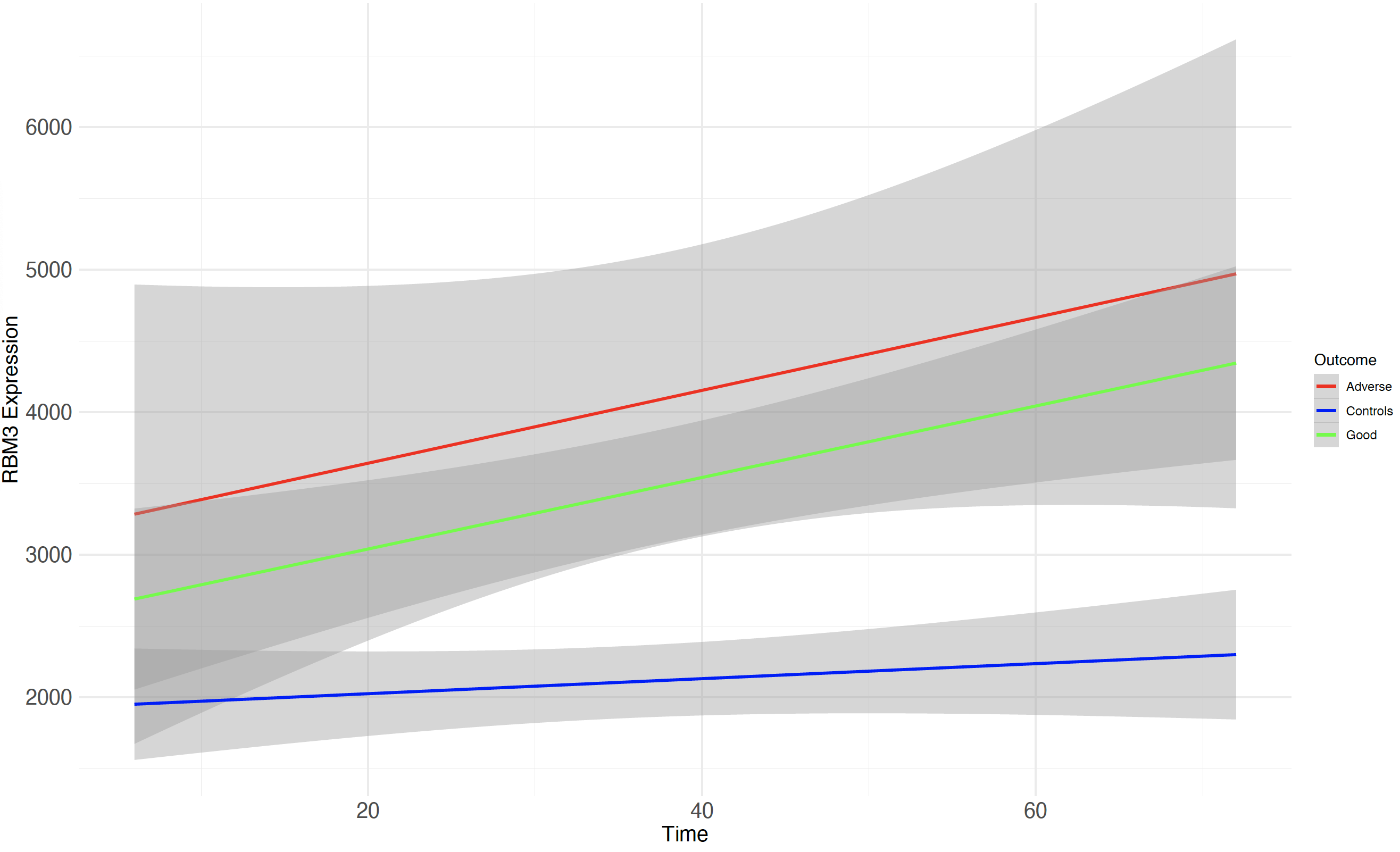
Design/Methods: We performed RNA Next Generation Sequencing (NGS) on whole-blood RNA from 35 HIE and 14 healthy control neonates, who had serial blood samples at 4 time points – a) within 6h after birth [T0]), b) 24h [T1], c) 48h [T2] and d) 72h [T3]). HIE neonates had whole-body hypothermia (33.5°C) throughout T1 to T3. Negative binomial generalized log-linear models were fitted using DESeq2 (v1.36.0) to identify significantly (FDR < 0.05) differentially expressed genes between HIE and healthy controls at each time point, with a log2 fold change cut-off of 1. Models were adjusted for gender, gestational age, and birth weight. Raw gene count was plotted for RBM3 in A) HIE and healthy controls, and B) moderate and severe HIE. A linear mixed-effects model was used to investigate the association between RBM3 expression over time according to the outcome group. Adverse outcome was defined as death/moderate/severe disability (Bayley III) at 18 months.
Results: A total of 262, 932, 3114, 1433 significant genes were identified at T0, T1, T2 and T3, respectively. At T0, RBM3 was not identified as being significantly different between HIE and controls. At T1, RBM3 was the third most significant gene (FDR < 0.0001, log2 fold change 1.1), and became the first most significant gene at T2 (FDR < 0.0001, log2 fold change 1.1) (Fig1). Despite not being within the 3 most significant genes at T3, RBM3 still figured amongst the top 20 (FDR < 0.0001, log2 fold change 1.1). RBM3 gene count was higher in HIE compared to controls at all time points, with a peak at T2 (Fig2A). Its expression was similar in moderate and severe HIE at all time points (Fig2B), indicating that RBM3 upregulation is likely to be related to hypothermia rather than disease severity. No significant difference was found when comparing RBM3 expression over the time between the adverse and good outcome groups (p=0.36), although significance was reached when comparing both outcome groups to controls (both p< 0.05) (Fig3).
Conclusion(s): RBM3 expression before initiation of whole-body hypothermia was similar in HIE and healthy controls, followed by an upregulation in HIE at 24, 48, and 72h. No association with adverse outcomes was found. RBM3 expression may be useful in the monitoring of response to hypothermia in HIE.
.png)