Neonatology
Session: Neonatal Neurology 1: Clinical
10 - Mattress temperatures during therapeutic hypothermia differ by cooling device but show intrinsic consistency in relation to outcome in infants with hypoxic-ischemic encephalopathy (HIE)
Friday, May 3, 2024
5:15 PM - 7:15 PM ET
Poster Number: 10
Publication Number: 10.450
Publication Number: 10.450

Thomas R. Wood, MD, PhD (he/him/his)
Assistant Professor
University of Washington School of Medicine
Seattle, Washington, United States
Presenting Author(s)
Background: As previously shown, in infants with moderate or severe hypoxic-ischemic encephalopathy (HIE) treated with therapeutic hypothermia (TH), the cooling mattress temperature (MT) required to achieve a core temperature of 33.5 C is a proxy of intrinsic temperature dysregulation and predicts long-term outcomes. However, cooling device technology varies by manufacturer and direct comparison of devices to assess generalizability of MT as biomarker is needed.
Objective: To examine if the MT required to achieve a core temperature of 33.5C is comparable across different cooling devices in a standardized cohort of neonates with moderate or severe HIE.
Design/Methods: In this cohort study, 40 infants from 3 study sites of the High-Dose Erythropoietin for Asphyxia and encephaLopathy (HEAL) Trial were cooled with 3 different devices: Blanketrol III (n=21), Tecotherm (n=12), or Arctic Sun (n=7). MT data were collected for every minute across the 72h cooling period, and average MT calculated for each infant within 6h cooling epochs after target temperature was reached. Neurodevelopmental impairment (NDI) was defined as mild, moderate or severe (Fig 1). MTs were compared by HIE severity, outcome, and device using linear mixed models with epoch, site, HIE severity and/or outcome as fixed effects and a random intercept for each infant.
Results: No or mild NDI was seen in 6 (86%), 6 (50%), and 21 (100%) of those cooled with the Arctic Sun, Tecotherm, and Blanketrol III, respectively. Due to the high incidence of no/mild NDI in two of the sites, the association between MT and outcome was confirmed within the cohort cooled with the Tecotherm, where infants with no/mild NDI had MT -2.5C (95% CI 0.1-5.0C, p=0.04) lower compared to those who experienced death/moderate-severe NDI (Fig 2). To determine whether different MTs are seen in infants with the same outcome cooled with different devices, MTs by device were compared in infants who experienced no/mild NDI only (n=33; Tecotherm n=6, Arctic Sun n=6, Blanketrol III n= 21). After adjusting for HIE severity and cooling epoch, MT from all three devices showed a similar trajectory (Fig 3A) but absolute temperatures differed between devices: the Arctic Sun (-3.6C; 95%CI -6.0 to -1.6C, p=0.008) and Tecotherm (-2.1C; 95%CI -4.5 to 0.3C, p=0.1) had lower MTs in the first 36h of cooling compared to the Blanketrol III (Fig 3B).
Conclusion(s): MT during TH is a promising biomarker of long-term outcome in infants with HIE, but absolute temperatures differ by cooling device. Therefore device-specific predictive cut-offs need to be generated and validated.
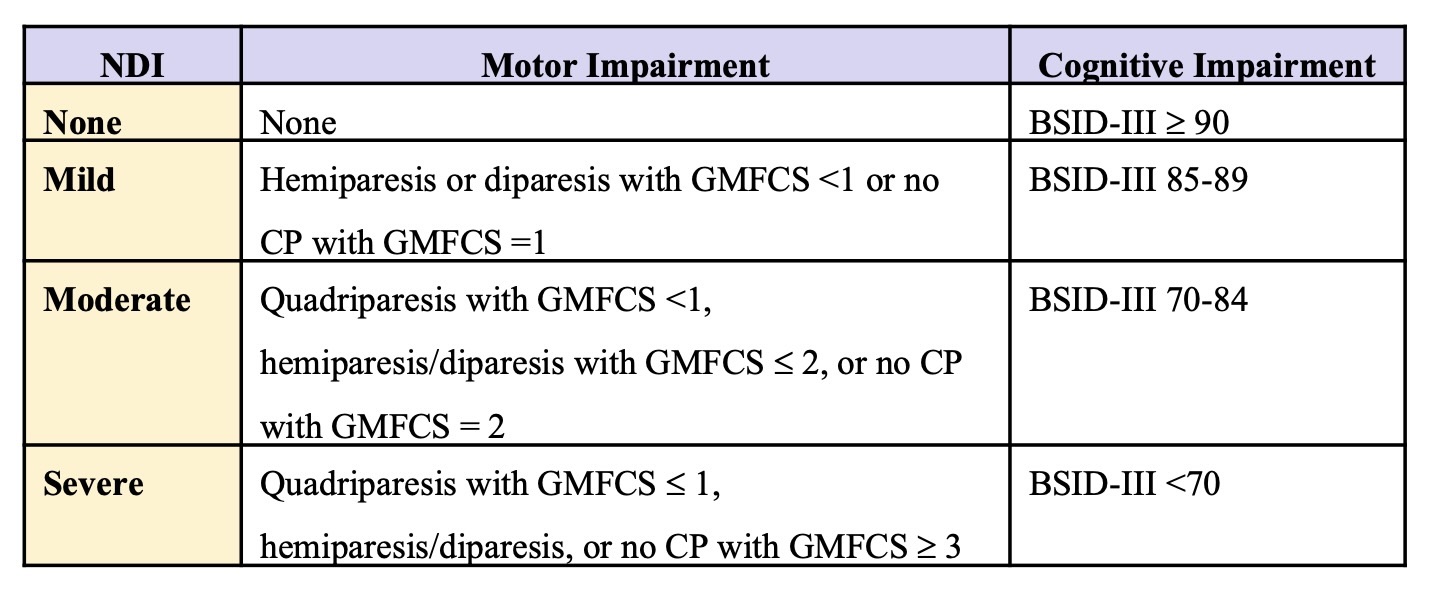
Figure 2 Temp.jpeg
Figure 3 Temp.jpeg
