Neonatology
Session: Neonatal Neurology 10: Neurodevelopment
357 - Modeling the neurodevelopmental gene, Eukaryotic Initiation Factor-4A2 (EIF4A2), in zebrafish
Monday, May 6, 2024
9:30 AM - 11:30 AM ET
Poster Number: 357
Publication Number: 357.2874
Publication Number: 357.2874

Anna R. Duncan, MD, MHS (she/her/hers)
Instructor of Pediatrics
MassGeneral Hospital for Children and Harvard Medical School
Boston, Massachusetts, United States
Presenting Author(s)
Background: The DEAD-box family of RNA helicases is critical for neurodevelopment. We have previously shown that variants in the DEAD-box encoding gene Eukaryotic Initiation Factor-4A2 (EIF4A2) lead to a neurodevelopmental disorder (NDD) characterized by intellectual disability, epilepsy, and structural changes in the brain (Paul, Duncan, et al, AJHG, 2023). The specific mechanisms by which EIF4A2 alters neurodevelopment, however, remain largely unknown. EIF4A2 is an essential regulator of protein translation that interacts with multiple critical genes during development. These include SOX2 and ARX, which are essential for interneuron differentiation and migration, suggesting that EIF4A2 serves a critical role in interneuron development as well.
Objective: To model EIF4A2 loss-of-function in zebrafish and to determine its impact on neurodevelopment.
Design/Methods: To understand the importance of EIF4A2 in neurodevelopment, we created eif4a2-/- zebrafish with CRISPR/Cas9 gene editing. Since individuals with variants in EIF4A2 have intractable seizures and motor delays, we assessed for hyperexcitability and motor function in wild type (WT) vs. eif4a2 -/- zebrafish. We also generated acute knockouts in transgenic zebrafish with GFP-labeled GABAergic interneurons.
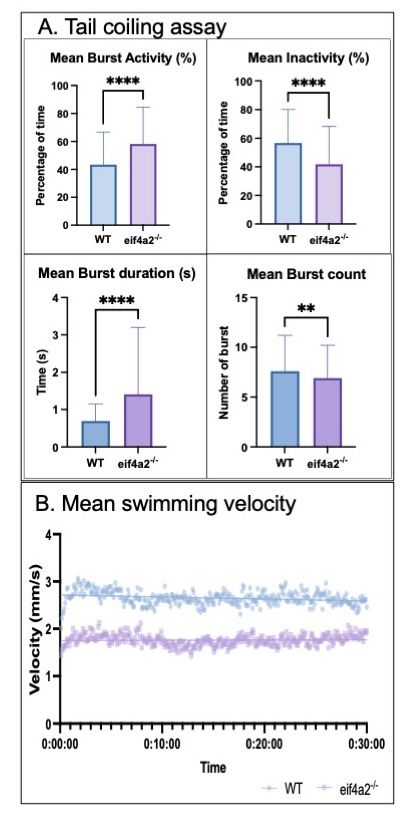
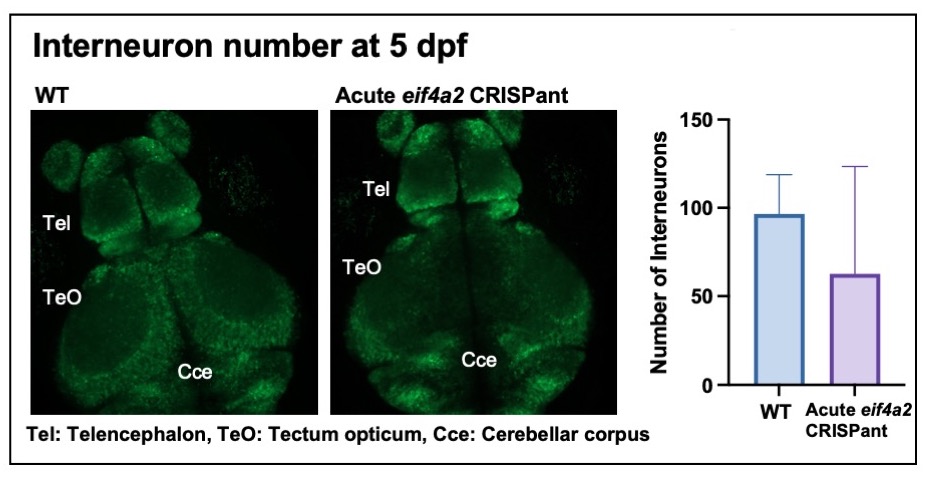
Results: We characterized motor behaviors with tail coiling and swim assays. In eif4a2-/- zebrafish, there was a significant increase in duration of tail coil bursts vs. WT at 17-24 hours post fertilization (hpf) (WT n=337, eif4a2-/- n=440, p< 0.01), suggesting hyperexcitability as early as 17 hpf. At 5 days post fertilization (dpf), the eif4a2-/- zebrafish consistently swam more slowly than WT (WT n=360, eif4a2-/- n=360, p< 0.0001). Since a majority of individuals with EIF4A2 variants have intractable seizures, type II seizures were recorded in zebrafish at 6 dpf. Eif4a2-/- zebrafish had increased spontaneous seizures vs. WT (WT n=185, eif4a2-/- n=314, p< 0.05). In order to understand how loss of eif4a2 impacts interneuron development, we generated acute CRISPR knockouts of eif4a2. Preliminary observations at 5 dpf demonstrate a reduction in interneurons in the eif4a2 acute CRISPants vs. WT (WT n=6, acute eif4a2 CRISPants n=10, p=0.22).
Conclusion(s): Similar to the individuals with pathogenic variants in EIF4A2, eif4a2-/- zebrafish exhibit hyperexcitability and motor impairments. Preliminary studies suggest that GABA interneurons may be impacted by loss of EIF4A2 function, suggesting that variants in EIF4A2 may lead to a novel interneuronopathy. Further data are needed to understand the impact of EIF4A2 on interneuron development.
PAS Figure 1_EIF4A2 CRISPR model.jpeg