Nephrology
Session: Nephrology 4
30 - Urinary Biomarkers in Childhood Nephrotic Syndrome: A Pilot Study
Sunday, May 5, 2024
3:30 PM - 6:00 PM ET
Poster Number: 30
Publication Number: 30.2196
Publication Number: 30.2196

Jessamyn S. Carter, MD, MS (she/her/hers)
Clinical Assistant Professor
University of Michigan Medical School
Ann Arbor, Michigan, United States
Presenting Author(s)
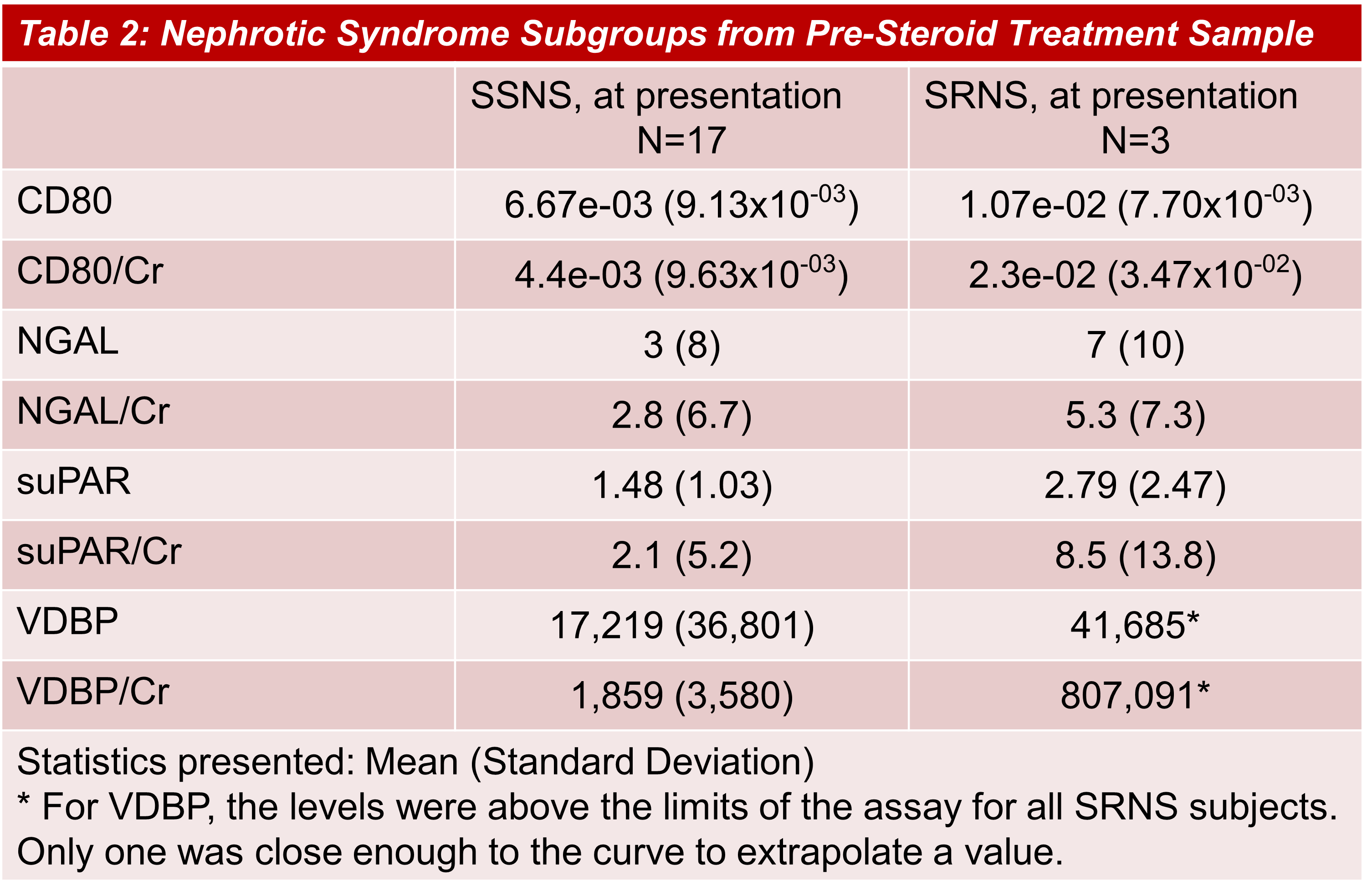
Background: Steroid resistant nephrotic syndrome is the most common acquired cause of end-stage kidney disease in childhood. Corticosteroids are the first line treatment of childhood nephrotic syndrome (NS), however, due to adverse effects of steroids, there is a need for markers to identify steroid resistance at disease presentation. Previous pilot studies identified candidate urinary biomarkers such as CD80, neutrophil gelatinase associated lipocalin (NGAL), soluble urokinase plasminogen receptor (suPAR), and vitamin D binding protein (VDBP). This study is uniquely designed to assess biomarkers at time of NS presentation and again after steroid treatment. Additionally, a non-NS proteinuria cohort is included to assess levels attributable to proteinuria alone.
Objective: Levels of candidate biomarkers will be compared amongst the different cohorts to evaluate if any result shows promise in the diagnosis and monitoring of childhood nephrotic syndrome.
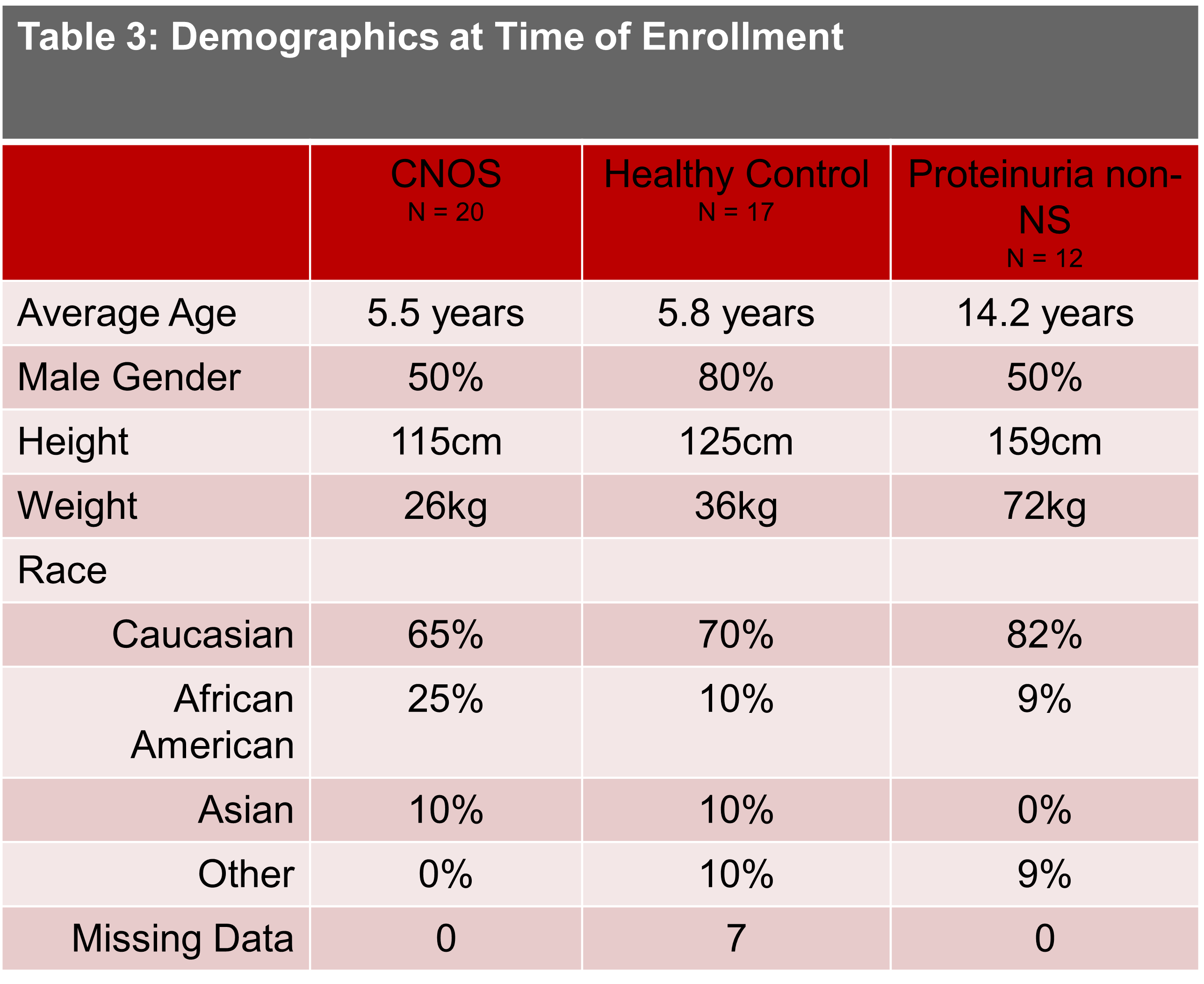
Design/Methods: Pre- and post-steroid urine samples from NS subjects were collected as part of the Childhood Nephrotic syndrome Observational Study (CNOS). Healthy controls and controls with proteinuria not due to NS were collected for comparison. Urine CD80, NGAL, suPAR, and VDBP were measured via Meso Scale Discovery. Values were standardized to urine creatinine measured by enzymatic assay.
Results: Urine CD80, suPAR, and VDBP were elevated in subjects with NS at presentation compared to healthy and to non-NS proteinuria subjects though none reached statistical difference. Levels decreased following initial steroid treatment in subjects with NS. A preliminary analysis of a small subgroup with steroid resistant NS showed a trend of further elevations, such as VDBP levels above assay limits.
Conclusion(s): Even accounting for small sample size, there is a trend towards a difference of biomarker levels at NS presentation compared to other cohorts and to NS subjects post-steroid course. This trend was most pronounced in a subgroup with steroid resistance. Evaluation in a larger cohort at time of NS presentation is warranted to clarify the diagnostic utility of these biomarkers.
.png)