Neonatology
Session: Neonatal Infectious Diseases/Immunology 3
624 - Large-Scale Implementation of Pooled Saliva Tests for Universal Screening of Congenital Cytomegalovirus Infection
Friday, May 3, 2024
5:15 PM - 7:15 PM ET
Poster Number: 624
Publication Number: 624.156
Publication Number: 624.156
- SE
Smadar Eventov-Friedman, MD PhD
Head of Department, Neonatology
Hadassah Medical Center and Faculty of Medicine, Hebrew University of Jerusalem, Israel
Jerusalem, Yerushalayim, Israel
Presenting Author(s)
Background: Targeted screening of congenital cytomegalovirus (cCMV) infection by saliva PCR, followed by urine-test confirmation of positive saliva results, is currently employed in many centers. However, targeted screening is known to miss the majority of congenitally infected infants who are asymptomatic at birth, yet are at risk for late-onset sequalae. Thus, universal newborn screening of cCMV has been increasingly advocated.
Objective: To assess a large-scale implementation of pooled saliva polymerase chain reaction (PCR) tests for universal screening of cCMV and to detect the actual efficiency (number of tests saved by pooling) and loss of sensitivity of the pooling, compared to individual-saliva testing.
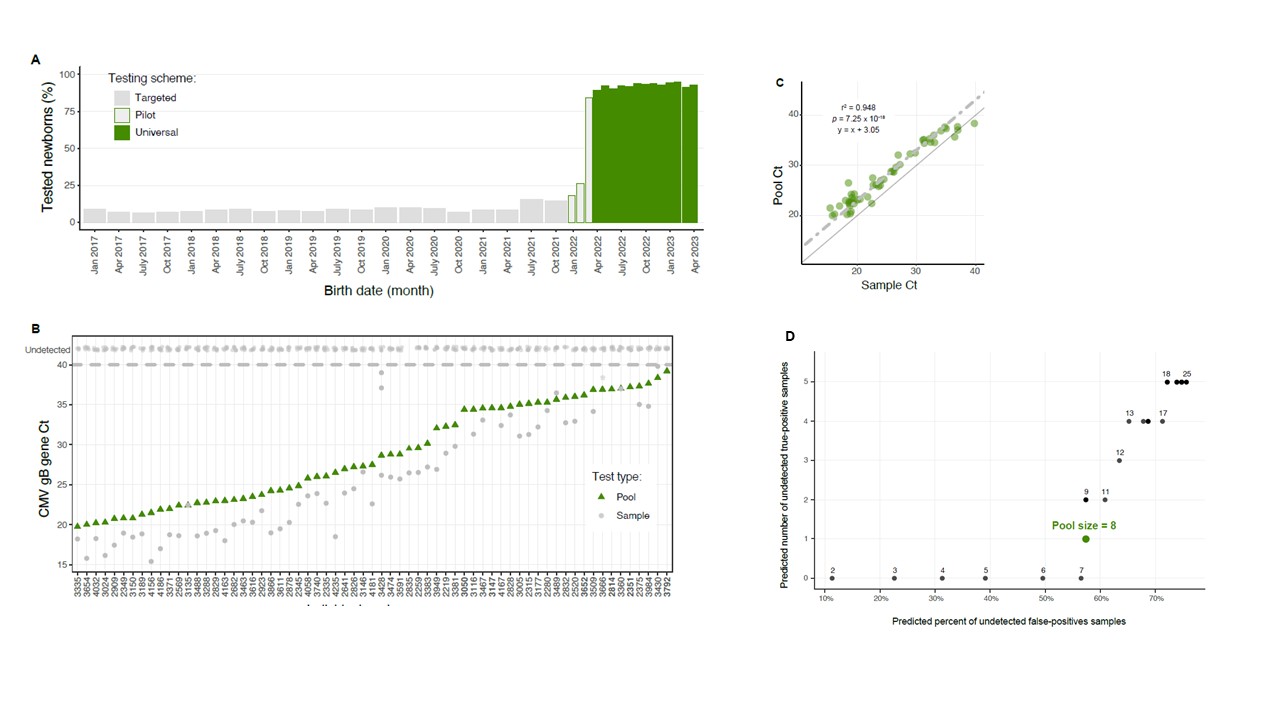
Design/Methods: In this prospective study, all newborns with parental consent were tested for cCMV. Following experimental validation, saliva samples were tested in 8-sample pools, all samples in negative pools were declared negative, and samples were retested individually only if the pool’s test result was positive. Positive saliva PCR results were confirmed by urine testing. Empirical pooling efficiency was calculated as the number of saliva samples tested per single PCR reaction.
Results: From April 2022 through April 2023, 15,805 newborns, constituting 94% of all live newborns at Hadassah Medical Center during this 13-month period, were screened for cCMV by pooled saliva tests. The high screening rate represented a drastic increase compared to the ~10% average screening rate during the targeted screening period. Our empirical efficiency was 6, thereby sparing ~83% of the saliva tests compared with individual testing, with only a minor, 3-PCR-cycle loss in actual sensitivity (Figure A-D). Overall, 54 infants were identified with cCMV out of 15,805 screened, with a birth prevalence of 3.4 per 1000 (95% CI, 2.6-4.3). More than half (55.6% ;30/54) of the congenitally infected infants identified by universal screening would have been missed by targeted screening. Studies are currently underway to examine the rate and risk factors for early and late complications of cCMV.
Conclusion(s): The data presented project on the wide feasibility and benefits of pooled saliva testing as an efficient, cost-sparing, and sensitive approach for universal screening of cCMV. Beyond the direct clinical implications, the implemented universal screening will define the burden, risk factors, and clinical outcomes of cCMV and increase awareness of this underrecognized congenital infection.