Quality Improvement/Patient Safety
Session: Quality Improvement/Patient Safety 4
29 - Use of Quality Improvement Tools to Increase Consent Rates in a Randomized Clinical Trial.
Monday, May 6, 2024
9:30 AM - 11:30 AM ET
Poster Number: 29
Publication Number: 29.3227
Publication Number: 29.3227

Sarah B. Kandil, MD (she/her/hers)
Associate Professor of Pediatrics, Critical Care Medicine
Yale School of Medicine
New Haven, Connecticut, United States
Presenting Author(s)
Background: Randomized clinical trials (RCT) are imperative to improve the care of critically ill children. Yet, recruitment poses unique challenges in this population, including the use of a surrogate decision-makers for consent, and tight enrollment windows when these decision-makers are severely stressed. These can lead to delays in trial completion, increase costs, and could potentially impact evidence-based care for children.
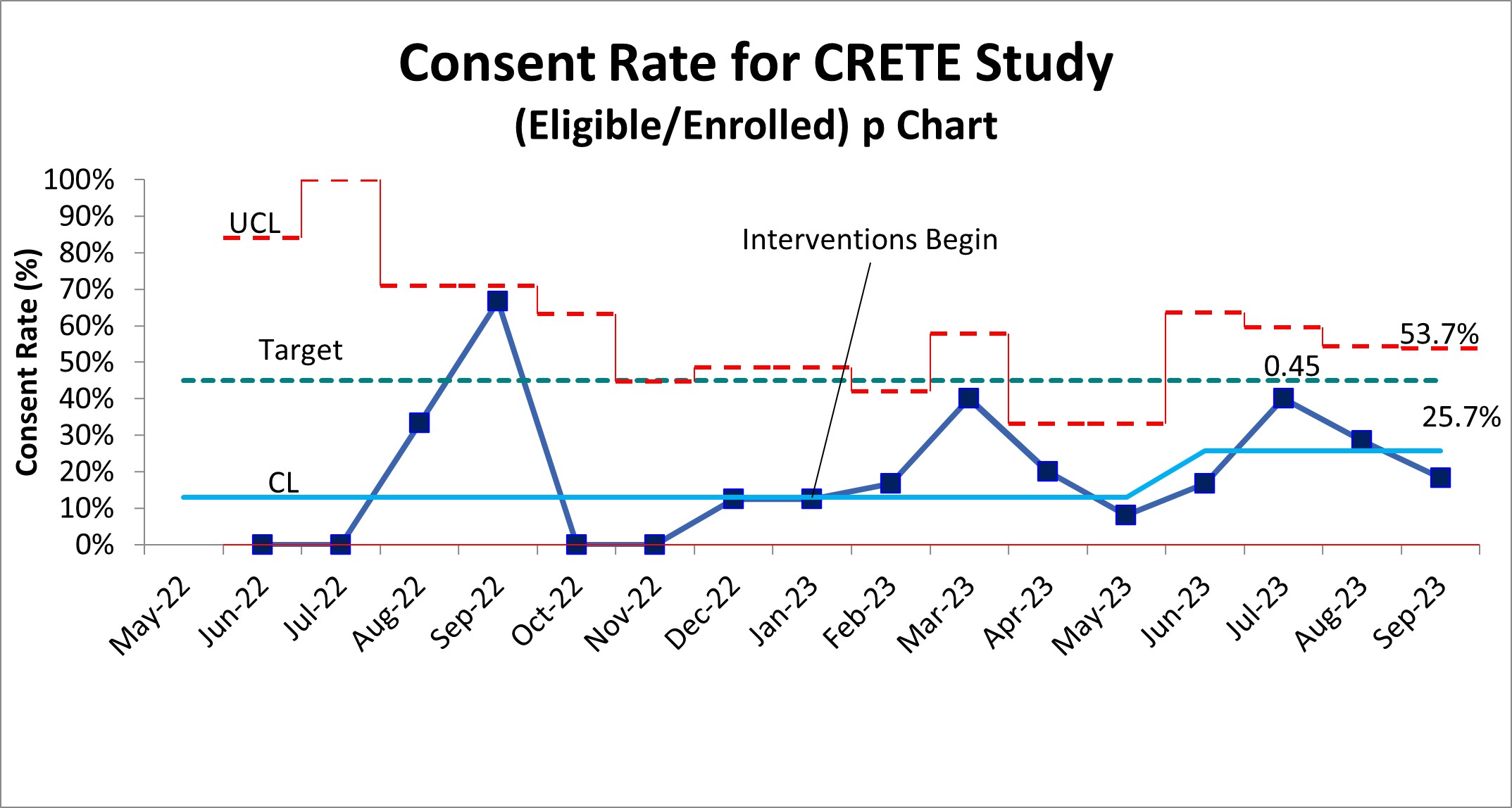
Objective: To increase consent rate in a multicenter RCT from 12% to 45% over 24 months using quality improvement (QI) methodology.
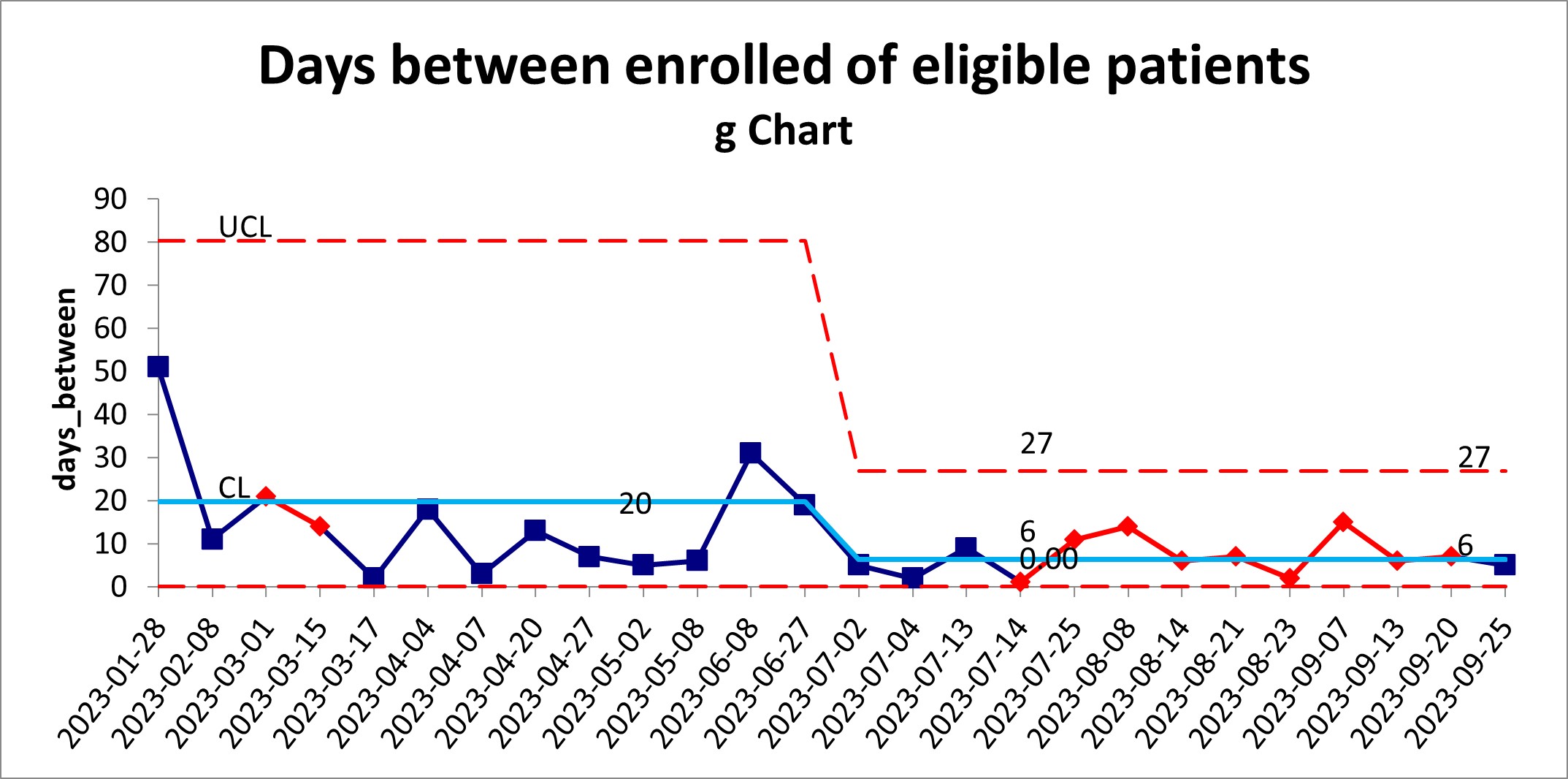
Design/Methods: We engaged key stakeholders from the Catheter-Related Early Thromboprophylaxis with Enoxaparin (CRETE) Studies (NCT04924322). This multicenter RCT’s goal is to determine the efficacy of early thromboprophylaxis against catheter-related thrombosis in critically ill children. Additional sites were added to increase enrollment, and both barriers and subsequent key drivers were identified to address consent. Initial drivers were communication, staffing shortages, and buy-in of local staff. In January 2023, we began education about the trial, standardized scripts, and shared communication tips with local staff. Iterations of these processes continued with onboarding of new study sites and research staff. Statistical process control charts were used to track consent rates, however, to detect shifts earlier we utilized the aggregate point rule (APR) and G charts.
Results: Initial consent rates across centers remained low at 12.9% from initiation of the trial in May 2022 to January 2023. We increased consent rates from 12.9% to 25.7%, with a shift (using the APR) in May 2023 (Figure 1). The G chart also demonstrated a decrease in days between enrollment of 20 days to 6 days or at least 1 patient/week (Figure 2) beginning June 2023. Further evaluation at site specific level is ongoing to determine best practices.
Conclusion(s): Using QI tools within a pediatric clinical trial, we have demonstrated improvement in enrollment. Use of the APR and G-chart allowed for earlier detection of improvement. QI methodology may help in future design of studies, practice, and education for those involved in research enrollment.