Nephrology
Session: Nephrology 3
8 - Lumasiran for Primary Hyperoxaluria Type 1: Analysis of Urinary Oxalate and eGFR Over Time in Patients With and Without Baseline Pyridoxine Use
Sunday, May 5, 2024
3:30 PM - 6:00 PM ET
Poster Number: 8
Publication Number: 8.1805
Publication Number: 8.1805

David J. Sas, DO, MPH (he/him/his)
Chief, Pediatric Nephrology and Hypertension
Mayo Clinic
Rochester, Minnesota, United States
Presenting Author(s)
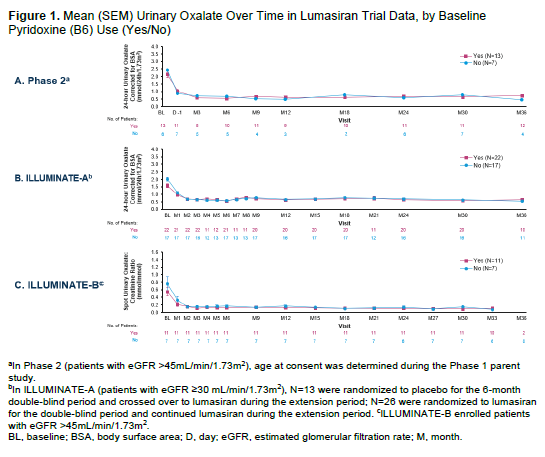
Background: Lumasiran is an RNA interference therapeutic that reduces hepatic oxalate overproduction in patients with primary hyperoxaluria type 1 (PH1) by reducing glycolate oxidase levels in the hepatocyte. Sustained reductions in urinary oxalate (UOx) have been demonstrated in multiple clinical trials of lumasiran. In ILLUMINATE-A, at 6 months, no meaningful difference in 24-hour UOx reduction was noted between patients with and without baseline vitamin B6 ([B6]; pyridoxine) use. Here, we present up to 3 years of UOx and kidney function results based on baseline B6 status.
Objective: To evaluate changes in UOx and estimated glomerular filtration rate (eGFR) by baseline B6 usage in lumasiran trials.
Design/Methods: Post hoc analysis of data reported to date for lumasiran trials enrolling patients with PH1 (Phase 2 [patient age ≥6 years; NCT03350451], Phase 3 ILLUMINATE-A [age ≥6 years; NCT03681184], and Phase 3 ILLUMINATE-B [age < 6 years; NCT03905694]) evaluating eGFR in patients with and without baseline B6 use.
Results: Baseline characteristics are shown in Table 1. Overall, 10% to 28% of enrolled patients had a B6-responsive genotype and were receiving B6 treatment at baseline; most patients with a B6-responsive genotype were taking B6 at baseline (65%-100%). Of the patients taking B6 at baseline, 77%, 91%, and 55% remained on B6 at the latest data cutoff for Phase 2, ILLUMINATE-A, and ILLUMINATE-B, respectively; among those not using B6 at baseline, none initiated B6 during the studies. Both B6-use and non-B6-use groups achieved similar rapid and sustained reductions in 24-hour UOx or spot UOx:creatinine over time (Figure 1). In general, eGFR remained stable over time regardless of baseline B6 use. Mean changes from baseline in eGFR at 1 year, 2 years, and Month 30/Month 36 were low (range, −8.0 to +5.4 mL/min/1.73m2) in B6-use and non-B6-use groups (Figure 2). In the Phase 2 and ILLUMINATE-A studies, eGFR levels were lower at baseline and overall in patients with versus without baseline B6 use; these differences may be due to chance given the relatively small study groups.
Conclusion(s): In a post hoc analysis of data from a Phase 2 and two Phase 3 trials, patients who were or were not receiving B6 at baseline had similar and sustained reductions in UOx after initiation of lumasiran, and eGFR was largely unchanged over time in both groups.
.png)
